Abstract
Aim:
This study aims to determine treatment patterns, long-term intraocular pressure (IOP) and perimetric control in different glaucomas seen at a tertiary eye center.
Settings and Design:
Hospital-based, cross-sectional chart review of patients routinely following up at an outpatient glaucoma service.
Methods:
Patients with a follow-up of at least 10 years were evaluated. Their mean IOP, visual field (VF) status, and medications/surgery required at final assessment were noted.
Statistical Analysis:
Descriptive statistics (mean, standard deviation, and range) were used for all parameters.
Results:
A total of 230 patients met our inclusion and exclusion criteria, 79 having ocular hypertension with open angles or primary angle closure (PAC), 35 primary open angle glaucoma (POAG), 50 PAC glaucoma (PACG), 20 primary congenital glaucoma (PCG), 46 secondary glaucoma patients. Ocular hypertensives with open angles showed progression to POAG in 3.7%, those with PAC in 5.2%, at a mean IOP of 17.3 ± 3.37 mmHg and 17.13 ± 4.41 mmHg, respectively. A progression on Humphrey Field Analyzer was seen in 11% of POAG and PACG eyes at a mean IOP of 13.50 ± 5.07 and 13.09 ± 3.95 mmHg, respectively. Fifteen percent of primary congenital glaucomas (PCGs) showed a glaucomatous VF defect after 10 years. In secondary glaucoma eyes, the mean IOP at last follow-up visit was 12.38 ± 3.74 mmHg, with progression noted in 7.69% of eyes.
Conclusion:
This study provides evidence that routine delivery of care can provide well controlled IOP in glaucomas, both primary and secondary, and the VF stabilized in about 90% of patients over a period of 10 years, with the currently available glaucoma medications and trabeculectomy.
Key words: 10-year follow-up, ocular hypertension, primary angle closure, primary angle closure glaucoma, primary congenital glaucoma, primary open angle glaucoma, progression in glaucoma, secondary glaucoma
Glaucoma accounts for 10% of blindness worldwide and 12.8% of blindness in India. It is estimated that by 2020, 40% cases of glaucoma worldwide will be in India and China alone.[1,2] The reported prevalence of glaucoma in India ranges from 2.6% to 4.1% in adults.[3,4] In children, primary congenital glaucoma (PCG), glaucoma associated with other congenital anomalies and secondary glaucomas are most frequent.[5] The prevalence of blindness in children has been estimated to be around 6.5/10,000, 4.2% of this attributable to PCG. In a hospital-based study in North India,[6] secondary glaucomas constituted 21.84% of all patients referred to the Glaucoma service. The incidence of secondary glaucoma in the Hooghly river glaucoma study was 0.15% in the urban population and 0.10% in the rural cohort.
There are many long-term studies on patients having primary open angle glaucoma (POAG), especially from developed countries, but few long-term studies on different glaucomas from India. This real world evaluation for over 10 years was undertaken to provide treatment patterns and outcomes in India.
This is a cross-sectional review of intraocular pressure (IOP), medications, frequency of surgery required and progression analysis at last review, in consecutive glaucoma patients, who were on regular follow-up for at least 10 years.
Methods
Clinical data of consecutive patients routinely reviewing at an outpatient glaucoma service with a single consultant, RS, were screened, and those with a follow-up of at least 10 years were evaluated.
At presentation, a detailed ocular history was obtained and all underwent slit lamp biomicroscopy, optical pachymetry, applanation tonometry, gonioscopy, and 30-2 program on Humphrey visual field analyzer (HFA).
In adults age >40 years, POAG was recorded in the presence of open angles on gonioscopy, and in chronic primary angle closure glaucoma (PACG), an occludable angle, having at least 180° of synechial closure on indentation/manipulative gonioscopy after iridotomy.
Common criteria for the diagnosis of all primary and secondary glaucomas were IOP >22 mmHg on at least three separate occasions and glaucomatous optic neuropathy consistent with VF loss in at least one eye. Glaucomatous disc changes were defined as a vertical cup to disc (C:D) ratio of >0.7, asymmetry of >0.2 between the two eyes, neuroretinal rim changes consisting of pallor or localized notching, and presence of retinal nerve fiber layer defects, in the absence of any other ocular or neurological pathology.
Congenital glaucoma was diagnosed by a history of photophobia, corneal clouding or tearing, associated with an IOP of >20 mmHg, enlarged corneal diameters and a C:D ratio of >0.4 on examination under anesthesia.
All primary angle closure (PAC)/PACG eyes underwent a neodymium:yttrium-aluminum-garnet iridotomy at presentation. Medical therapy was prescribed to attain target IOP as per clinical practice, taking into account, baseline IOP, the severity of glaucomatous damage, – mean deviation (MD) <6 dB, 6–12 dB, >12 dB, systemic diseases, age, etc., The “target” IOP for ocular hypertensives was <18 mmHg, mild to moderate glaucoma – 14–16 mmHg, and severe glaucoma – 10–12 mmHg. The target for congenital glaucoma was <15 mmHg. Surgery was undertaken for patients with an inadequate control of IOP on maximum tolerated medical therapy.
Patients were reviewed every 3/6 months depending on the severity of glaucomatous optic neuropathy, IOP within “target” range and any change in VF loss. Only those patients, who followed up for at least 10 years with a minimum of 1 visit in a year, were included in the final analysis. Patients who had not reviewed at least once per year and those who could not perform reliable perimetry or tonometry were excluded from this study.
Full threshold/SITA standard 30-2 program, standard automated perimetry was performed using Humphrey Field Analyzer (HFA) (Model 750, Carl Zeiss Meditec, Dublin CA, USA). Progression was confirmed when three consecutive, reliable fields showed a reproducible change in at least three locations, on guided progression analysis (GPA).
Statistics
The patients were analyzed in subgroups by diagnosis. Descriptive statistics – mean, standard deviation and range, were ascertained for all parameters. Progression or stabilization on GPA was noted.[7] To be labeled as progressors, the patient had to have a rate of progression significant at P < 0.05, and at least three points significantly depressed on three consecutive fields. Patients with severe glaucoma had an overview of their 10-2 printout evaluated. The mean IOP, VF status and medications/surgery required at final assessment in each group of glaucomatous eyes, were noted. SPSS software version 17.5 (SPSS, Inc., Chicago, Illinois) was used for the analysis.
Results
A total of 1951 patients who were on routine follow-up were screened over a 6-month period, at a tertiary outpatient glaucoma service. In total, 230 patients who were on regular follow-up for over 10 years and met our inclusion and exclusion criteria were analyzed. Seventy-nine having ocular hypertension with open angles or PAC, 35 POAG, 50 PACG, 20 PCG, 46 secondary glaucoma patients.
Ocular hypertension
Seventy-nine eyes of 79 patients were analyzed, with an average age of 66.3 ± 9.23 years years. There were 31 males and 48 females. Seventy-six percent of all ocular hypertensives were on one glaucoma medication alone, while 6% were on three medications at last review.
Ocular hypertension with an open angle
Twenty-seven patients had a mean baseline IOP of 26.04 ± 4.86 mmHg. All patients were on medical therapy, with a mean of IOP 17.33 ± 3.37 mmHg at last review. Definite progression on HFA was seen in one patient, 3.7%.
Primary angle closure with ocular hypertension
Fifty-two patients having a mean baseline IOP of 25.8 ± 4.64 mmHg. All patients were on medical therapy, mean IOP of 17.13 ± 4.41 mmHg. Definite progression on HFA was seen in three patients, 5.2%.
Primary open angle glaucoma
Ten-year data of 35 patients, 69 eyes, having POAG were studied. One eye had neovascular glaucoma and was excluded. The mean age of POAG patients at last review was 68.12 ± 9.03 years, (range: 49–82 years). The male to female ratio was 2.5:1. The average follow-up duration in this group was 13.8 ± 4.58 years, (range: 10–29 years). The baseline IOP off treatment, where available in the records was 24.7 ± 4.2 mmHg. The mean IOP at last follow-up visit was 13.50 ± 5.07 mmHg, with 59 eyes having an IOP of <14 mmHg and those with only early glaucomatous VF loss <18 mmHg. Twenty of 69, 28.9%, eyes underwent trabeculectomy. The average number of topical glaucoma drugs in the medical management group at the last follow-up was 1.92 ± 1.35. The average number of medications being used in the trabeculectomy group was 0.85 ± 1.12 with a mean follow-up duration after trabeculectomy being 10.28 ± 5.90 years (range: 3–26 years). Progression on GPA of HFA was noted in 8 out 69 eyes, 11.59%, over the total duration of follow-up. Four eyes had a significant depression in 3 loci and 4 eyes in >4 loci.
Primary angle closure glaucoma
Fifty patients of PACG, 97 eyes, were analyzed. Two eyes in the absolute stage at presentation and 1 with neovascular glaucoma were excluded. The mean age of patients at last review was 68.96 ± 8.50 years (range: 49–84 years). Male: female ratio was 2:1. The average follow-up duration in this group was 14.52 ± 4.38 years (range: 10–27 years). The baseline IOP off treatment, where available in the records was 29.15 ± 7.9 mmHg. Mean IOP at last follow-up visit was 13.09 ± 3.95 mmHg. Seventy-five eyes with moderate to severe glaucomatous optic neuropathy had an IOP of <14 mmHg, 22 eyes with early Glaucoma had an IOP of 16–20 mmHg. The average number of topical glaucoma drugs in the medical management group at last follow-up was 2.11 ± 0.79. 25 of 97, 25.8%, eyes underwent a trabeculectomy. The average number of medications being used in the trabeculectomy group was 0.44 ± 0.71, with mean follow-up duration after trabeculectomy being 8.81 ± 6.10 years (range: 1–23 years). The progression on GPA of HFA was noted in 11 out 97 eyes, 11.34%, over the total duration of follow-up. Eight eyes had a significant depression in 3 loci and three eyes in >3 loci.
Congenital glaucoma
Twenty patients (40 eyes) of congenital glaucoma on follow-up for over 10 years were reviewed during these 6 months. The mean age of the patients at last follow-up was 15.95 ± 5.75 years (range: 10–30 years). Male:female ratio was 1.85:1. All patients in this group underwent surgery in the 32 affected eyes immediately at presentation, 8 of 40 eyes required medication alone and were well controlled on one or two glaucoma drugs. Average follow-up duration since surgery in this group was – 16.05 ± 5.75 years. All 32 eyes underwent a combined trabeculectomy with trabeculectomy with mitomycin C (MMC). Mean IOP at last follow-up in eyes undergoing surgery was 13.10 ± 4.67 mmHg. 27 eyes had an IOP of <14 mmHg, and 13 eyes had an IOP of 16–18 mmHg. The average number of medications being used at the last follow-up in the surgical group was 0.78 ± 0.90. The mean IOP in eyes with unoperated congenital glaucoma was 15.5 ± 3.66 mmHg. The average number of medications being used in the unoperated eyes was 1.37 ± 0.91. Progression on perimetry could not be ascertained as no baseline perimetry was available, however, only six eyes, 15%, showed a glaucomatous VF defect after 10 years, both hemispheres being involved in a patient with Axenfeld–Rieger syndrome, and a single hemisphere, in the rest.
Secondary glaucomas
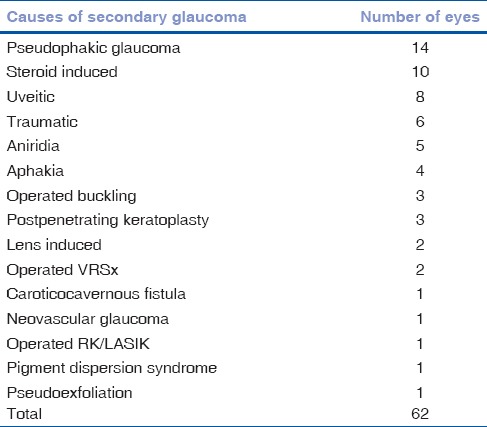
The causes of secondary glaucoma in this group have been listed in Table 1. Forty-six patients, 62 eyes, having secondary glaucoma due to various causes were analyzed. Five eyes at absolute stage of glaucoma on presentation were excluded from the analysis, three neovascular glaucoma, one operated vitreoretinal surgery, and one aniridia. The mean age of patients in this group was 53.08 ± 20.9 years (range: 18–90 years). The male:female ratio was 2.06:1. Average follow-up duration in this group was 15.43 ± 5.51 years (range: 10–34 years). The baseline IOP off treatment, where available in the records was 34.3 ± 6.5 mmHg. The mean IOP at last follow-up visit was 12.38 ± 3.74 mmHg. Fifty eyes had an IOP of <14 mmHg, 10 eyes had an IOP up to 18 mmHg. The average number of topical glaucoma drugs in the medical management group at last follow-up was 1.66 ± 0.79. 25 of 62, 40.3%, eyes underwent a trabeculectomy. The average number of medications being used in the trabeculectomy group was 0.88 ± 1.17 with mean follow-up duration after trabeculectomy being 12.52 ± 5.44 years (range: 5–27 years). Progression on HFA was noted in 2 of 26 eyes, 7.69%, where HFA was possible, over a review of at least 10 years.
Table 1.
Causes of secondary glaucoma and their frequency
Discussion
Glaucoma is a chronic progressive disease that has to be treated and reviewed lifelong. It is, therefore, important to know what is likely to happen to patients with different types of glaucoma in the long-term. There are large randomized trials which have reported long-term results in largely POAG.[8] However, there are few long-term Indian studies, and none looking at a follow-up of 10 years on routine clinical care, the “real” world. This study was undertaken to provide some data on treatment patterns and outcomes so that patients could be counseled accordingly.
Progression in treated ocular hypertensive eyes with open angles in this review was seen in 3.7% over 10 years, as compared to 5.2% in PAC eyes with ocular hypertension. The difference was not statistically significant. The ocular hypertension treatment (OHT) study lowered IOP by 20%, mean IOP of 19.3 mmHg, and reported 4.4% progression in treated eyes at 5 years, and at 10-years they noticed that progression in the high-risk group decreased from 42% to 19% and in the lowest-risk, from 7% to 4%.[8] Sihota et al.[9] recorded a progression of PAC that became ocular hypertensives to developing PACG in 8 of 26 eyes, 31%, reflecting a subgroup having progressive trabecular dysfunction. This study looked at all ocular hypertensives, i.e., those at the high end of physiological IOP, as well as those with trabecular dysfunction.
This study found that 11% of POAG eyes progressed over more than 10 years of follow-up, with a mean IOP of 13.5 ± 5.07 mmHg. The Early Manifest Glaucoma Trial[10] lowered IOP by 25% and reported progression over 6 years in 45% of eyes that were treated with betaxolol and trabeculoplasty, and if required, additional Xalatan. In this study, the IOP was reduced to a mean of 15.5 mmHg. The collaborative initial glaucoma study reported progression over 8 years, in 25.5% and 21.3% of patients treated with medications or surgery, respectively.[8] Patients in whom the IOP was lowered by 38%–46% did not progress. The advanced glaucoma intervention study lowered IOP to 15.1–16.7 mmHg, and recorded a 30% rate of perimetric progression.[8]
Our review noted a progression on perimetry in 11% of PACG eyes over more than 10 years; none became blind. Quek et al.[11] studied 57 PACG eyes, and noted that over 10 years, a third of PACG patients were found to have VF progression, with 7% progressing to blindness while on treatment. Rao et al.[12] studied 68 eyes in which a laser iridotomy was performed, for 9 PAC and 59 PACG patients. Presenting IOP and highest recorded IOP before were the most significant predictors of the need for additional treatment including trabeculectomy. Sihota et al.[9] found that 8 of 72 PAC eyes, 11%, developed VF defects on standard achromatic perimetry, over a mean period of 6.89 ± 2.4 years. Eyes that progressed on perimetry had a significantly narrower angle recess, ≤10°, and a higher baseline MD and pattern standard deviation, respectively. They also had a larger intervisit IOP fluctuation of 8.9 ± 2.3 mmHg, of stable eyes, 6.2 ± 3 mmHg, and a longer duration of follow-up.
Rao et al.[13] reported that severity of VF damage at presentation and long-term IOP fluctuation were associated with increased rate of progression. Greater IOP fluctuation was seen in eyes later undergoing glaucoma surgery and those requiring more glaucoma medications during follow-up. Vijaya et al.[14] reported that of 133 subjects having pseudoexfoliation reexamined at 6 years, 8, 6.0%, developed glaucoma and all had OHT at baseline.
In this study, patients with PCG with a “target” IOP of <15 mmHg, had a mean IOP after more than 10 years, in eyes undergoing surgery of 13.10 ± 4.67 mmHg. Only 15% of eyes showed a glaucomatous VF loss. Mandal et al.[15] kept a target IOP of <21 mmHg, with a success rate was 85.2, 80.4, 77.2, 72.6, 66.2, and 57.5% at 1st, 2nd, 3rd, 4th, 5th, and 6th years, respectively. Perimetric data were not available. Ramkrishanan et al.[16] described overall complete surgical success in pediatric glaucomas as 73.3%, qualified success in 16.8%, and failure in 10.7% at 1 year.
Secondary glaucomas seen over this period in our study were commonly pseudophakic, steroid induced, traumatic, and uveitic. About 40% of patients required a trabeculectomy, and all patients had an IOP of <18 mmHg at 10 years. An earlier study[17] noted that topical steroid use (73.5%) was the most frequent cause of glaucoma. The mean baseline IOP in eyes requiring surgery was 49.67 ± 13.28 mmHg and in eyes managed medically was 30.36 ± 7.51 mmHg. Patients with steroid-induced glaucoma, who were ≤20 years old, with a higher IOP, and greater glaucomatous optic neuropathy, were more likely to need surgery. Gupta et al.[18] studied 27 patients having a postvitreoretinal surgery secondary glaucoma, with a mean age of 28.3 ± 15.2 years. All eyes underwent a superior Ahmed glaucoma valve implantation, with a 37% success at 5 years, and 48% rate of complications, 22% of which were vision-threatening.
Keeping in mind the socioeconomic circumstances in India, it is important to know that expenditure on glaucoma medications[19] ranged from 0.3% of their monthly gross income in high-income group to 123% in a low-income group. The total expenditure including travel, stay, and loss of wages of patients and accompanying persons ranged from 1.6% in high-income group to 137% of the monthly income in the low-income group. Ninety-two percent were not covered by any insurance plan/government reimbursement for their treatment. Therefore, glaucoma filtering surgery should be kept as an early option as long-term success of trabeculectomy in this study in all glaucomas was good with 0.44–0.85 medications being required over 10 years. Previous Indian studies[20,21] have also shown a good medium term success of a trabeculectomy, even with low-dose MMC. A mean IOP at 2 years of 11.1 ± 1.6 mmHg and 10.8 ± 2.8 mmHg, with a mean reduction in IOP of 50.6 ± 1.23%, and 53.7 ± 2.25% was noted using MMC 0.1 mg/ml or 0.2 mg/ml, respectively for 1 min subconjunctivally.
The probable reason for lower progression rates in almost all subgroups of glaucoma in this study, as compared to randomized control trials was the lower “target” IOP. The randomized trials largely set a specific percentage reduction as the treatment goal or were using less effective medications than those currently available. In our protocol, the “target” IOP was set to <18 mmHg for ocular hypertensives, 14–16 mmHg for patients having mild to moderate glaucomatous optic neuropathy and 10–12 mmHg for those having VF loss in both hemispheres, severe field loss.
This was a cross-sectional clinical review of routine care in the real world, a “snapshot” of a patient, providing only some outcomes. A limitation of our study was that patients living near the hospital, socioeconomically able to come regularly and those doing well were possibly more likely to review for 10 years. Another limitation was that patients with poor vision who could not perform perimetry were excluded. A single IOP record at final review does not indicate changes over time; however, all patients were on review with one consultant who ensured that target IOP was maintained over visits.
Conclusion
This study provides evidence that routine delivery of care can provide well controlled IOP in all glaucomas, both primary and secondary, and the VF stabilized in about 90% of patients over a period of 10 years, with the currently available glaucoma medications and trabeculectomy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thylefors B, Négrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull World Health Organ. 1995;73:115–21. [PMC free article] [PubMed] [Google Scholar]
- 3.Ramakrishnan R, Nirmalan PK, Krishnadas R, Thulasiraj RD, Tielsch JM, Katz J, et al. Glaucoma in a rural population of Southern India: The Aravind comprehensive eye survey. Ophthalmology. 2003;110:1484–90. doi: 10.1016/S0161-6420(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 4.Dandona L, Dandona R, Srinivas M, Mandal P, John RK, McCarty CA, et al. Open-angle glaucoma in an urban population in Southern India: The Andhra Pradesh eye disease study. Ophthalmology. 2000;107:1702–9. doi: 10.1016/s0161-6420(00)00275-x. [DOI] [PubMed] [Google Scholar]
- 5.Dandona L, Williams JD, Williams BC, Rao GN. Population-based assessment of childhood blindness in Southern India. Arch Ophthalmol. 1998;116:545–6. [PubMed] [Google Scholar]
- 6.Gadia R, Sihota R, Dada T, Gupta V. Current profile of secondary glaucomas. Indian J Ophthalmol. 2008;56:285–9. doi: 10.4103/0301-4738.41411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artes PH, O'Leary N, Nicolela MT, Chauhan BC, Crabb DP. Visual field progression in glaucoma: What is the specificity of the Guided Progression Analysis? Ophthalmology. 2014;121:2023–7. doi: 10.1016/j.ophtha.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Panarelli JF, Banitt MR, Sidoti PA, Budenz DL, Singh K. Clinical impact of 8 prospective, randomized, multicenter glaucoma trials. J Glaucoma. 2015;24:64–8. doi: 10.1097/IJG.0b013e318295200b. [DOI] [PubMed] [Google Scholar]
- 9.Sihota R, Rao A, Gupta V, Srinivasan G, Sharma A. Progression in primary angle closure eyes. J Glaucoma. 2010;19:632–6. doi: 10.1097/IJG.0b013e3181ca7de9. [DOI] [PubMed] [Google Scholar]
- 10.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. EMGT Group. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Quek DT, Koh VT, Tan GS, Perera SA, Wong TT, Aung T. Blindness and long-term progression of visual field defects in chinese patients with primary angle-closure glaucoma. Am J Ophthalmol. 2011;152:463–9. doi: 10.1016/j.ajo.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Rao A, Rao HL, Kumar AU, Babu JG, Madhulata U, Arthi J, et al. Outcomes of laser peripheral iridotomy in angle closure disease. Semin Ophthalmol. 2013;28:4–8. doi: 10.3109/08820538.2012.702260. [DOI] [PubMed] [Google Scholar]
- 13.Rao HL, Addepalli UK, Jonnadula GB, Kumbar T, Senthil S, Garudadri CS. Relationship between intraocular pressure and rate of visual field progression in treated glaucoma. J Glaucoma. 2013;22:719–24. doi: 10.1097/IJG.0b013e318259b0c2. [DOI] [PubMed] [Google Scholar]
- 14.Vijaya L, Asokan R, Panday M, Choudhari NS, Sathyamangalam RV, Velumuri L, et al. The prevalence of pseudoexfoliation and the long-term changes in eyes with pseudoexfoliation in a South Indian population. J Glaucoma. 2016;25:e596–602. doi: 10.1097/IJG.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 15.Mandal AK, Gothwal VK, Nutheti R. Surgical outcome of primary developmental glaucoma: A single surgeon's long-term experience from a tertiary eye care centre in India. Eye (Lond) 2007;21:764–74. doi: 10.1038/sj.eye.6702324. [DOI] [PubMed] [Google Scholar]
- 16.Ramkrishanan R, Mitra A, Abdul Kader M. Surgical and visual outcomes of childhood glaucoma at a tertiary eye care center in South India. Asia Pac J Ophthalmol (Phila) 2015;4:250–8. doi: 10.1097/APO.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 17.Sihota R, Konkal VL, Dada T, Agarwal HC, Singh R. Prospective, long-term evaluation of steroid-induced glaucoma. Eye (Lond) 2008;22:26–30. doi: 10.1038/sj.eye.6702474. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Chaurasia AK, Chawla R, Kapoor KS, Mahalingam K, Swamy DR, et al. Long-term outcomes of glaucoma drainage devices for glaucoma post-vitreoretinal surgery with silicone oil insertion: A prospective evaluation. Graefes Arch Clin Exp Ophthalmol. 2016;254:2449–54. doi: 10.1007/s00417-016-3469-9. [DOI] [PubMed] [Google Scholar]
- 19.Nayak B, Gupta S, Kumar G, Dada T, Gupta V, Sihota R. Socioeconomics of long-term glaucoma therapy in India. Indian J Ophthalmol. 2015;63:20–4. doi: 10.4103/0301-4738.151458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sihota R, Gupta V, Agarwal HC. Long-term evaluation of trabeculectomy in primary open angle glaucoma and chronic primary angle closure glaucoma in an Asian population. Clin Exp Ophthalmol. 2004;32:23–8. doi: 10.1046/j.1442-9071.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- 21.Sihota R, Angmo D, Chandra A, Gupta V, Sharma A, Pandey RM. Evaluating the long-term efficacy of short-duration 0.1 mg/ml and 0.2 mg/ml MMC in primary trabeculectomy for primary adult glaucoma. Graefes Arch Clin Exp Ophthalmol. 2015;253:1153–9. doi: 10.1007/s00417-015-3028-9. [DOI] [PubMed] [Google Scholar]


