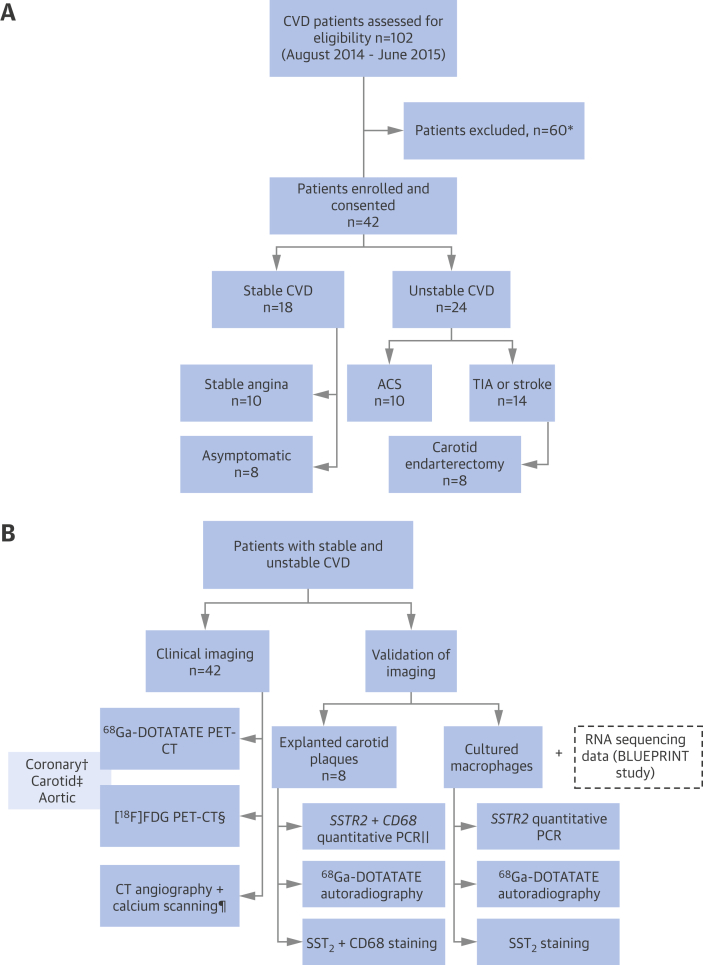
Figure 1.
The VISION Study
Patient (A) and procedure (B) flowcharts. ∗Did not meet study criteria, n = 8; other clinical factors, n = 3; declined/cancelled, n = 49. †Coronary artery PET data excluded in ACS patients with ambiguous culprit arteries (n = 2). ‡Carotid artery PET data excluded in patients with prior carotid surgery (n = 2). §[18F]-FDG PET imaging not completed because of timing of surgery (n = 1). ‖Tissue samples excluded owing to insufficient mRNA extracted for quantitative PCR (n = 2). ¶CT scans not completed (calcium scan, n = 1; coronary angiogram, n = 5; carotid angiogram, n = 2). ACS = acute coronary syndrome; CT = computed tomography; CVD = cardiovascular disease; FDG = fluorodeoxyglucose; PCR = polymerase chain reaction; PET = positron emission tomography; TIA = transient ischemic attack; VISION = Vascular Inflammation imaging using Somatostatin receptor positron emissION tomography.