Fig. 7.
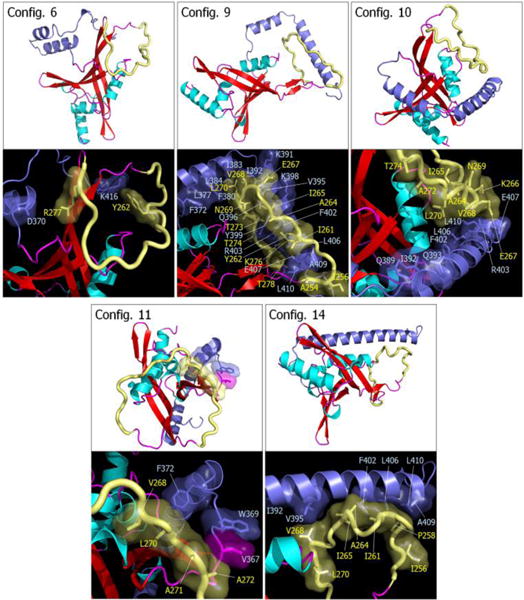
Snapshots representing average conformations of RASSF5. Selected RASSF5 configurations 6, 9, 10, 11, and 14 are shown on the top of each panel. In the cartoon representing the secondary structure, the α-helix and β-sheet structures are colored cyan and red, respectively. The SARAH domain is highlighted by blue, and the flexible loop is shown as yellow tube. Highlighted interfaces between SARAH and flexible loop are shown in the bottom of each panel. Residues involving the interactions are marked.
