Abstract
Objective
Human milk is universally accounted as the preeminent source of nutrition for infants. Surprisingly, no approved diagnostic tests are available for the diagnosis of physical condition of the breast. Somatic cell count (SCC) is a key tool commonly used in the dairy industry to provide evidence of udder health, which in turn determines the quality of bovine and cattle milk. Elevated levels of somatic cells in milk are observed during intra-mammary infectious state in bovine animals, which is due to active participation of the immune system. This constraint in humans can principally be used to study breast health.
Materials and Methods
In the present study, 176 breast milk samples in total were randomly collected from four different regions of Gujarat, India. All the samples were subjected to somatic cell count and total bacterial count tests. The effect of geographical region and maternal health was studied on the basis of milk SCC and total bacterial load. Statistical interpretation of the results was done using PRISM 6.07.
Results
Breast showing clinical symptoms of mastitis yielded a high SCC (>104 cells/microliter (μL)) and bacterial count (between 105 to 1011 Colony Forming Unit (CFU)/milliliter (mL)) in comparison to milk collected from healthy breast (<104 cells/μL and 103 to 104 CFU/mL). Statistical analysis reveals existence of significant correlation between the geographical region and SCC count of milk collected from healthy breast whereas no correlation was observed in infected breast milk. The study has also demonstrated that a lineer correlation exists between SCC and abundance of bacteria present in breast milk.
Conclusion
The present study could be employed to predict lactating breast health.
Keywords: Breast milk, breast health, colony forming unit, somatic cell count
Introduction
Human milk is a dynamic and bioactive fluid, which undergoes changes in composition, i.e., from colostrum to late lactation, and also varies from mother to mother (1). Remarkably, the lactating mother breast is one of the metabolically important organs for which no medical tests exist to prove its health status. Milk consists of somatic cells that are 75% leukocytes, i.e., neutrophils, erythrocytes, macrophages, lymphocytes and 25% epithelial cells. During intra-mammary infection, a significant increase in total SCC is observed where mostly epithelial cells and white blood cells are present in large numbers. It is also observed that the cell count increases whenever the mammary glands are injured.
The white blood cells play a fundamental role in the immune system. They are considered as a defending element, which fights against infection and are actively involved in the repair of damaged tissue. During inflammation, a considerable number of neutrophils is reported in the milk, i.e., accounting for more than 90% of the total SCC (2). Alteration in neutrophil count is also dependent on the lactational stages and breast health in addition to the prevalence of pathogens (3–5). Recent studies illustrate that increase in a number of leukocytes and macrophage is directly associated with breast infection and that their number decreases significantly upon recovery (6, 7). This means that the amount of cellular constituent in milk foretells the health of a mammary gland. Also, to some extent, infants’ respiratory or gastrointestinal infection contribute to the transmission of infection to the mother, thereby increasing the number of somatic cells and other immuno-components that fight against infection (6, 8).
Epithelial cells are the chief structural and functional constitutes of the breast, constituting 99% of the cellular component of breast milk (7, 8). Mammary epithelial cells (MEC), which are milk-secreting cells of the mammary gland, play a unique functional role within the breast tissue. While most epithelial layers in the body serve a protective function, MECs selectively take up components of the plasma and manufacture constituents of breast milk to secrete a nutritive and immune-factor-rich fluid providing defense against any type of inflammation or infection (9).
At the early stages of infection, the predominant defense strategy that is rapidly induced is the innate immune response. This response is ubiquitous, short-acting and targets a range of microorganisms (3, 10, 11). Cells participating in innate response are the infiltrated neutrophils and macrophages; these are the cells which are recruited at the site of inflammation (12). Series of specific and nonspecific events occur, which involve the participation of cytokines and chemokines secreted by neutrophils and macrophages leading to the eradication of antigens (13, 14).
However, little is known about the factors involved in this rapid recruitment at the site of infection. Thus, the need arises to study the MECs, which are the primary cells that encounter the antigens invading a lactating breast. There is abundant evidence the demonstrating prominent role of MECs during initiation of innate immune response through triggering neutrophil infiltration and activation of innate immune cells leading to increase in milk SCC (8, 15).
Thus, the objective of the present study was to confirm the significant association among SCC, bacterial count and influences of geographical location on lactating mother. The study will help conduct research into lactating breast health.
Materials and Methods
Study Population
Volunteers, lactating mothers aged between 20 to 30 years, were selected for the collection of milk with their prior consent. In total, 176 milk samples (88 from healthy breast milk and 88 from infected breast milk) were collected between postpartum days 15 and 90. Details of lactating mothers who participated in the study as sorted by geographical regions are given in (Table 1) The volunteers belonging to the same geographical area were placed in one group. Expertise of an experienced gynecologist was used to differentiate between healthy and infected breasts. Written consent forms were obtained from by all volunteer mothers that narrated history of health status, mode of delivery, medication and recurrent symptoms in case of an infected mother. All the infected mother that were selected for the study had common symptoms like breast engorgement, fever, redness on the breast, pus draining from the nipples and all the samples were collected before the administration of any treatment and medication. Also, milk was collected from women 20 to 30 days after the appearance of infection symptoms.
Table 1.
Distribution of sample collection according to city and number
| Sr. no. | City name | Community | Latitude and longitude | No. of samples collection | |
|---|---|---|---|---|---|
|
| |||||
| Healthy breast milk | Infected breast milk | ||||
| 1 | Anand | Urban | 22.5645° N, 72.9289° E | 22 | 22 |
| 2 | Chikhli | Rural | 20.7579° N, 73.0632° E | 22 | 22 |
| 3 | Valsad | Rural | 20.4925° N, 73.1350° E | 22 | 22 |
| 4 | Dharmpur | Rural | 20.5401° N, 73.1792° E | 22 | 22 |
Collection of Samples
Before the collection of milk, mammary areola and nipples were cleaned with soap and sterile water followed by swabbing with sanitizer (Himedia) (16). The first few drops of milk were discarded following the collection of milk in a sterile Falcon tube by manual expression. The collected samples were kept at 4°C until transportation to the laboratory. Human milk sample collection protocol was approved by the human ethics committee (Approval No-IEC-3/GJPIASR/2015–16/E/3).
Examination of Somatic Cells in Breast Milk
A SCC was performed using an electronic somatic cell counter (Foss, Hillerød Denmark).
Bacteriological Analysis of Breast Milk
Milk samples were diluted ten times using water for injection and plated on nutrient agar plate (Himedia), then incubated at 37°C aerobically for 24 hours. Bacteria were quantified based on colony forming unit (CFU).
Statistical Analysis
Experimental data obtained were entered in a spreadsheet and exported to a statistical package (Prism 6.07, GraphPad Software, Inc, San Diego, California, USA) for statistical analysis (17). Student t-test was performed in order to find the relation between SCC of healthy and infected breast milk, followed by analysis of variance (ANOVA) to know the effect of geographical region on SCC of healthy and infected breast milk. Further correlation between SCC and bacterial abundance in the milk was analyzed using paired t-test and linear regression analysis (18).
Results
Effect of Breast Condition in SCC
Intra-mammary health can be foreseen by performing a SCC. SCC represents the most profound and effective way to check the breast health. It reflects the response of the immune system to intra-mammary infection where an increase SCC is observed that is <103 cells/μL (healthy breast) to >104 cells/μL (infected breast) (Figure 1). In this study, we compared the mean SCC between the group of healthy mother’s breast milk and the group of infected mother’s breast milk using unpaired two tail t-test. The test assumes that variances for the two populations are the same. The t-statistic is 10.3946, which is greater than the t critical value (tc = 1.976) at 174 degrees of freedom. The absolute value of the calculated t exceeds the critical value (10.3946>1.976); hence, the value of SCC of healthy human milk and infected human milk are significantly different. Using the P-value approach, the corresponding two-tailed p-value was found to be 0.00001, which is less than 0.05 (Table 2). We have concluded that the mean SCC values of healthy mother’s breast milk and infected mother’s breast milk are different, which reveals that SCC is affected by breast condition. Box plot graphs exemplify the response generated by the whole group based on which we can visualize the range and characteristics of responses for a large group. In our study, SCC in healthy milk was in the range of 16–614 cells/μL whereas SCC in infected breast milk was in the range of 572–17588 cells/μL.
Figure 1.
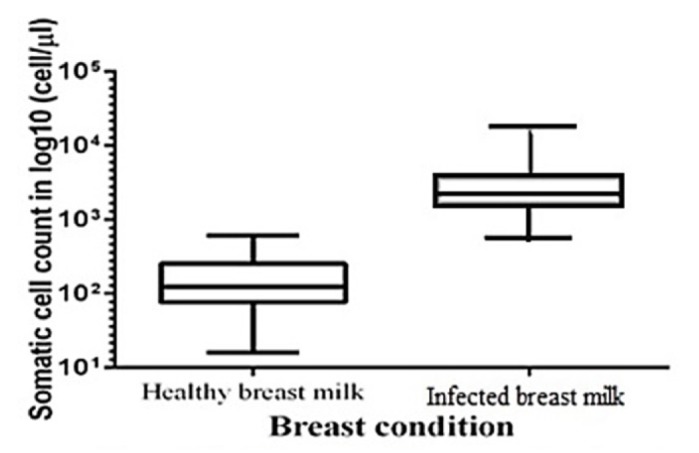
Effect of breast condition on somatic cell count
Table 2.
Effect of breast health status on somatic cell count (SCC)
| Breast condition | Mean | Variance | Stand. Dev. | N | t-value | Degrees of freedom | Critical value | p (< 0.05) |
|---|---|---|---|---|---|---|---|---|
| Healthy breast milk | 186.9659 | 24196.2402 | 155.5514 | 88 | 10.3946 | 175 | 1.976 | 0.00001 |
| Infected breast milk | 3604.0909 | 9485967.8997 | 3079.9299 | 88 |
Correlation Between Geographical Region and Somatic Cell Count of Healthy and Infected Breast Milk Using ANOVA Test
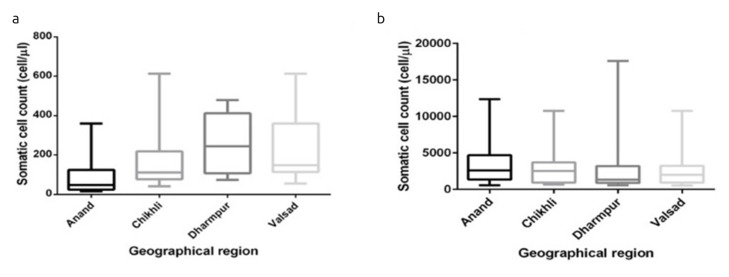
An ANOVA test performed for healthy breast milk revealed that F-statistic (6.245) is greater than the critical value of F (Fc) (F=6.245>Fc=2.713), which meant that SCC of healthy human milk was different when collected from four region. When a P-value analysis approach was adopted, the p-value obtained was 0.0007, which was less than 0.05 (i.e. degree of freedom). This specifies that the population mean value of SCC for all four regions is not same at the 0.05 significance level (Table 3). Column statistics analysis reveals that the media of all four geographical regions are not closer to one other. Tail of box plot also reveals higher variability in SCC, Chikhli showing extreme variation followed by Valsad and Dharmpur (Figure 2a). Similarly, when a one-way ANOVA test was performed for the infected breast milk, it was observed that F-statistic was 0.322, which was less than F critical value, i.e., 2.713. This indicates that there is no significant difference observed in SCC of infected breast milk collected from four regions. Also, the P value obtained was 0.8094, which was greater than 0.05, indicating that the SCC value was same in all the infected milk samples (Table 4). The media of all four regions were found to be closer to one other after performing column statistics. Also, the box plot showed no significant variability in SCC; however, the sample collected from Dharmpur showed more variability than remaining three regions (Figure 2b). Thus, after performing the statistical analysis of somatic cells collected from infected and healthy human milk, it was observed that geographical regions influenced the SCC values of healthy milk whereas similar type of SCC were observed in the infected milk sample, which did not indicate any significant difference.
Table 3.
Correlation between geographical region and somatic cell count of healthy milk by One-way ANOVA test
| Group of geographical region | degrees of freedom (DF) | sum of squares (SS) | mean sum of squares (MS) | F-statistic | p (< 0.05) |
|---|---|---|---|---|---|
| Within the group | 3 | 1,721,206 | 20,490.548 | 6.245 | 0.001 |
| Between the group | 84 | 383,871.3 | 127,957.103 | ||
| Total | 87 | 2,105,077.3 |
DF: degrees of freedom; SS: sum of squares; MS: mean sum of squares
Figure 2. a, b.
Effect of geographical region on somatic cell count (a) healthy breast milk and (b) infected breast milk
Table 4.
Effect of geographical region on somatic cell count of infected mother breast milk by One-way ANOVA test
| Group of geographical region | degrees of freedom (DF) | sum of squares (SS) | mean sum of squares (MS) | F-statistic | p |
|---|---|---|---|---|---|
| Within the group | 3 | 891,261,311.214 | 10,610,253.705 | 0.322 | 0.809 |
| Between the group | 84 | 10,253,339.151 | 3,417,779.717 | ||
| Total | 87 | 901,514,650.365 |
Correlation Between Somatic Cell Count And Microbial Abundance (CFU) in Breast Milk
As a purpose of total bacterial count, all the samples were spread on nutrient agar medium. After 24 hours of incubation, CFUs were calculated. Afterwards, the data generated were subjected to a linear correlation analysis. Figure 3 indicates a positive linear relationship that developed between SCC and the total count of microorganisms (Table 5). The numbers of microorganisms were increased by increasing the SCC. In infected breast milk, the CFU increased with a rapid rise in SCC- i.e., from 105 to 1011 CFU/mL. The abundance of microbes in milk is reflected by the presence of somatic cells in milk. In healthy milk, the total bacterial count was observed to be in the range of 2× 103 to 1.4×104 CFU/mL while CFU increase was in the range of 105 to 1011 CFU/mL for infected milk. Milk with low SCC reflected good maternal health. Also, Murphy et al. (19), Barbano et al. (20), and Malinowski et al. (21) investigated the relationship between the number of somatic cells and the mastitis causing microbes and reported similar results in this respect.
Figure 3.
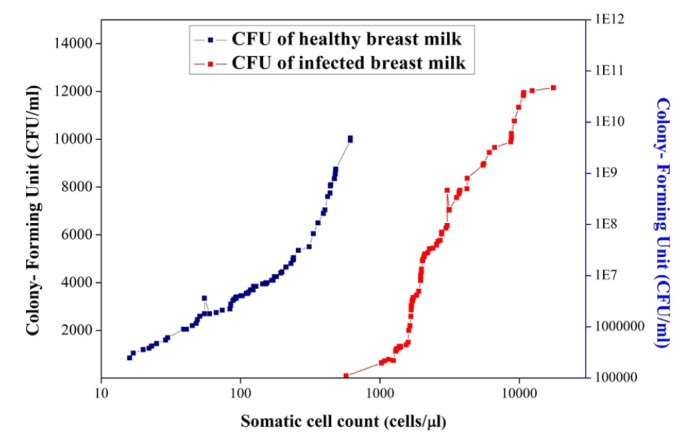
Relation between somatic cells and number of microorganisms in healthy breast milk
Table 5.
Relation between somatic cell count and colony forming unit
| p | < 0.0001 |
| Significantly different? (P < 0.05) | Yes |
| R squared | 0.5803 |
| Correlation coefficient (r) | 0.9778 |
R squared = coefficient of determination
Discussion
The outcome of this study verifies that infected breast milk shows increased SCC compared to healthy breast (22). At the beginning of an inflammatory incidence, one of the prime responses of the innate immune cascade is to stimulate an influx of immune cells (mainly neutrophils) at the site of infection in the mammary gland (11). The measurement of somatic cells (immune cell) in the milk is normally used in the dairy industry to assess the severity of mammary inflammation. The concentration of cytokine (IL-8) triggers the infiltration of immune cells, which causes increase in the number of immune cells during inflammation observed both in bovine and human milk. The four key points of the inflammatory immune system are: (1) phagocytes and their secreted interferons and cytokines; (2) cell-mediated immunity composed of natural killer cells (NK), T-cells and secreted proteins which regulate, stimulate and inhibit the immune response like interferons and cytokines; (3) humoral immunity containing immunoglobulins, B-cells and plasma cells; (4) the complement cascade. Although discretely deliberated, there are extensive and complex interactions between the four above-mentioned immunological responses, leading to the development of synchronized and effectual immune response against virtually any human pathogens. A complex series of events occur in the body in response to the entry of antigens and against its specific virulence factors. Because of these defending mechanisms developed by the body, a sudden increase in the SCC is observed at the time of infection (23). In 2013, Hassiotou et al. (7) observed an increase in leucocytes count from 0.7% to 96.3% in infected breast milk and it was already known that somatic cells consisted of 75% leukocytes and 25% epithelial cells (24). As such, it is known that leukocytes play an important role in fighting infection and it is also known that increase in neutrophils is observed during bacterial infection, which is further increased during the inflammatory response causing the invasion of immune cell at the site of entry of the pathogen (7). Thus, leucocytes may cause indirect increase in SCCs. In case of dairy animals, infection of the udder leads to development of the mastitis, which is the second most infectious disease - i.e., next to foot-and-mouth disease. In the present study, it could be clearly observed from Figure 1 that the endpoint (maximum value- 614 cells/μL) of healthy breast milk SCC and starting point (lowest value: 572 cells/μL) of infected breast milk is close to one other. Based on that, we can calculate that 103 cells/μL is the count that distinguishes the SCC in healthy and infected breast milk. Healthy subjects in the present study were screened based upon visual signs and symptoms of bacterial infection and the health of the infant was also considered as it may act as a source for the spread of infection to the mother. Clinical signs and symptoms such as breast engorgement, fever, redness on the breast and pus draining from the nipples were taken into consideration while the infected mother was screened (25, 26). According to the International Dairy Federation guideline, animals and their milk are classified as subclinical mastitis, clinical mastitis and non-mastitic (normal) using the SCC threshold of 500 cells/μL, >5000 cells/μL and <200 cells/μL for clinical mastitis (27). In Europe, milk with SCC higher than 400 cells/μL cannot be used for human consumption, whereas the thresholds are 750 cells/μL and 500 cells/μL in the USA and Canada, respectively (28).
Our observations also suggest that the geographical region does not have a significant effect on the SCC of infected breast indicating that a similar type of immune response is generated by the infected mother. A research by Patel et al. (29) reveals the difference in the microbial community observed in infected breast milk and healthy breast milk. When microbes invade the breast, the defense mechanisms become activated; however, the level of immunity expresses very strong individuals to individuals. The hypothesis that geographical region has no significant correlations with the SCC of infected breast milk is supported by the ANOVA test. In terms of the geographical regions, Chikhli is classified as a rural area, where 97% rural population lives below the poverty line and is encircled by an unhygienic environment. In addition, Chikhli is the largest taluka of Gujarat state that has a diverse population, where only 53.77% of the females are literate (30). Hence, fostering mothers are less knowledgeable about sustaining hygienic condition, thus increasing the chance of breast infection by microbes leading to increased somatic cell count. Like Chikhli, Valsad also has mix kind of population and the environmental microflora is different due to industrialization. In contrast, Dharmpur is a small taluka typically populated by tribes with the least environmental variation (Figure 2a). Anand, which is a metropolitan town, has a rate of 72% literacy among mothers (30), who are very well awere of hygiene and sanitization. Moreover, Anand has a healthy environment, which in itself reduces the chance of infection.
There is ample evidence which articulates that human milk reflects a diverse and feasible microbial community, although it is secreted by a healthy lactating mother with no signs and symptom of infection or other mammary gland disease. Somehow, variations are observed in the microbial profiling, which may be due to behavioral, environmental or genetic differences or as a consequence of methodological variation (i.e. instrument, time of milk sample collection, maternal physiological and medical history). In 2003, Martin et al. (31) originally isolated lactic acid producing bacteria from healthy human milk. Furthermore, he also studied the abundance of microbes in breast milk collected from urban and rural habitats, which was 101 CFU/mL and 104 CFU/mL, respectively (31). On the other hand, to explore the healthy milk bacteria, a metagenomics approach was used by Hunt et al. The study reveals that the microbial abundance in healthy milk not only consist results heathy bacteria but also opportunistic microbes like Pseudomonas, Staphylococcus, Corynebacterium, Streptococcus, Serratia, Sphingomonas, Ralstonia, Bradyrhizobium that may be contributing to the development of minor immune response where the SCC count of log104 cells/μL can be observed (32). The paired t-test analysis of somatic cells and total bacterial counts of the same individual produces a positive linear correlation when elucidated, which suggests that SCC is amplified in parallel with increased microbial community in healthy human milk, The culture-dependent analysis of Indian infected mother breast milk is reported to be 5.0×108 CFU/mL on average (29). In 2015, Jimenez et al., (33) reported shotgun sequencing of 10 healthy and 10 mastitic breast milk samples, which revealed that microbial abundance in mastitis-infected mother was higher than that of the healthy lactating mother. Thus, based on culture-dependent and independent studies, it can be concluded that an infected mother has high abundance of bacteria resulting in high CFU/mL and the amount of somatic cell is also higher as a result of an action by the immune system. Therefore, SCC and CFU counts can be effectively used to predict lactating breast health.
Conclusion
It has long been known that human milk consists of maternal cells; however, less well-studied points include the number of somatic cells, their importance for the mother and infant as well as any factors affecting them. Naturally, it is believed that breast milk has immune cells and epithelial cells. In an effort to shed light on breast milk, we studied a population of breastfeeding mothers from four different regions having healthy and infected breast conditions. Statistical results reveal that intra-mammary infection causes significant increases in total SCC in human milk. The present study reveals that the correlation between the SCC and bacterial counts gradually increase in the SCC observed during breast infection. The importance of the current study cannot be neglected as breast infection during lactation is found to be more common with the subsequent development of mastitis. Early diagnosis is expected to be instrumental in effective treatment, thus allowing for a longer duration of breastfeeding and confirming the healthiest initiation for the infant along with multidimensional benefits to the mother. In the present study, statistical analysis reveals that mothers from urban regions have lower counts of somatic cells as compared to mothers from rural areas as they are more conscious about hygiene and also consume healthy food. Also, the geographical region shows a significant correlation with the SCC in a healthy mother; however, in case of an infected mother, no such correlation was observed due to the prevalence of a similar type of pathogen. Also, if breast infection is identified during the initial course, then the consequences could be overcome without precluding lactation and transmission of the disease can also be prevented. Our data emphasize that somatic cells are used as a diagnostic tool for the preliminary assessment of lactating breast health status, which could play a significant role for health in later stages of life.
Acknowledgments
The authors are thankful and gratified to Charutar Vidya Mandal (CVM) and Anand Agriculture University (AAU) for providing research platform.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Govindbhai Jorabhai Patel Ayurveda College and Surajben Govindbhai Patel Ayurveda Hospital (Approval No-IEC-3/GJPIASR/2015–16/E/39).
Informed Consent: Informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.K., Y.V., C.J.; Design - A.K., Y.V., C.J., S.P.; Supervision - A.K., Y.V., C.J., S.P.; Resource - A.K., Y.V., C.J., S.P., D.N.; Materials - Y.V., S.P.; Data Collection and/or Processing - A.K., Y.V., S.P.; Analysis and/or Interpretation: A.K., Y.V., C.J., S.P., D.N. Literature Search - A.K., Y.V., C.J., S.P., D.N.; Writing - A.K., Y.V.; Critical Reviews - A.K., Y.V., C.J., S.P., D.N.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Liu Z, Roy NC, Guo Y, Jia H, Ryan L, Samuelsson L, Thomas A, Plowman J, Clerens S, Day L, Young W. Human Breast Milk and Infant Formulas Differentially Modify the Intestinal Microbiota in Human Infants and Host Physiology in Rats. J Nutr. 2016;146:191–199. doi: 10.3945/jn.115.223552. https://doi.org/10.3945/jn.115.223552. [DOI] [PubMed] [Google Scholar]
- 2.Miller W, Scott W, Morris R, Fraser H, Sharpe R. Growth of human breast cancer cells inhibited by a luteinizing hormone-releasing hormone agonist. Nature. 1985;313:231–233. doi: 10.1038/313231a0. https://doi.org/10.1038/313231a0. [DOI] [PubMed] [Google Scholar]
- 3.Boutinaud M, Jammes H. Potential uses of milk epithelial cells: a review. Reprod Nutr Dev. 2002;42:133–47. doi: 10.1051/rnd:2002013. https://doi.org/10.1051/rnd:2002013. [DOI] [PubMed] [Google Scholar]
- 4.Hassiotou F, Trengove N, Tat Lai C, Filgueira L, Blancafort P, Hartmann P, editors. Breastmilk stem cells: An overview of the current knowledge. Breastfeeding and Lactation Symposium; Vienna: Austria. 2012. [Google Scholar]
- 5.Cregan MD. The paracellular pathway and the lactating human breast. University of Western; Australia: 2002. [Google Scholar]
- 6.Riskin A, Almog M, Peri R, Halasz K, Srugo I, Kessel A. Changes in immunomodulatory constituents of human milk in response to active infection in the nursing infant. Pediatr Res. 2011;71:220–225. doi: 10.1038/pr.2011.34. https://doi.org/10.1038/pr.2011.34. [DOI] [PubMed] [Google Scholar]
- 7.Hassiotou F, Hepworth AR, Metzger P, Lai CT, Trengove N, Hartmann PE, Filgueira L. Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clinical & translational immunology. 2013;2:e3. doi: 10.1038/cti.2013.1. https://doi.org/10.1038/cti.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassiotou F, Geddes D. Anatomy of the human mammary gland: Current status of knowledge. Clin Anat. 2013;26:29–48. doi: 10.1002/ca.22165. https://doi.org/10.1002/ca.22165. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence RA, Lawrence RM. Breastfeeding: A guide for the medical professional. Elsevier Health Sciences; 2010. [Google Scholar]
- 10.Burton JL, Erskine RJ. Immunity and mastitis some new ideas for an old disease. Veterinary Clinics: Food Animal Practice. 2003;19:1–45. doi: 10.1016/s0749-0720(02)00073-7. https://doi.org/10.1016/s0749-0720(02)00073-7. [DOI] [PubMed] [Google Scholar]
- 11.Rainard P, Riollet C. Innate immunity of the bovine mammary gland. Vet Res. 2006;37:369–400. doi: 10.1051/vetres:2006007. https://doi.org/10.1051/vetres:2006007. [DOI] [PubMed] [Google Scholar]
- 12.Bannerman DD, Paape MJ, Lee J-W, Zhao X, Hope JC, Rainard P. Escherichia coli and Staphylococcus aureus elicit differential innate immune responses following intramammary infection. Clin Diagn Lab Immunol. 2004;11:463–472. doi: 10.1128/CDLI.11.3.463-472.2004. https://doi.org/10.1128/cdli.11.3.463-472.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paape MJ, Shafer-Weaver K, Capuco AV, Van Oostveldt K, Burvenich C. Biology of the Mammary Gland. Springer; 2002. Immune surveillance of mammary tissue by phagocytic cells; pp. 259–277. [DOI] [PubMed] [Google Scholar]
- 14.Leitner G, Eligulashvily R, Krifucks O, Perl S, Saran A. Immune cell differentiation in mammary gland tissues and milk of cows chronically infected with Staphylococcus aureus. J Vet Med Series B. 2003;50:45–52. doi: 10.1046/j.1439-0450.2003.00602.x. https://doi.org/10.1046/j.1439-0450.2003.00602.x. [DOI] [PubMed] [Google Scholar]
- 15.Gray C, Strandberg Y, Donaldson L, Tellam RL. Bovine mammary epithelial cells, initiators of innate immune responses to mastitis. Anim Prod Sci. 2005;45:757–761. https://doi.org/10.1071/EA05046. [Google Scholar]
- 16.Vaidya Y, Patel S, Patel R, Joshi C, Kunjadia A. Exploring the microbiota of human milk using the culture-dependent method. Int j adv res. 2015;3:462–471. [Google Scholar]
- 17.Kwiatkowska M, Norman G, Parker D, editors. Computer aided verification. Springer; 2011. PRISM 4.0: Verification of probabilistic real-time systems. [Google Scholar]
- 18.Motulsky H. Prism 4 statistics guide—statistical analyses for laboratory and clinical researchers. GraphPad Software Inc; San Diego, CA: 2003. pp. 122–6. [Google Scholar]
- 19.Murphy S, Boor K. Sources and causes of high bacteria counts in raw milk: An abbreviated review. Dairy food environ sanit. 2000;20:1–4. [Google Scholar]
- 20.Barbano D. The role of milk quality and mastitis control in addressing future dairy food marketing opportunities in a global economy. Processing of the NMC Regional Meeting; 2004 june 29–30; Bloomington, Minnesota, USA. pp. 1–5. [Google Scholar]
- 21.Malinowski E, Lassa H, Klossowska A, Markiewicz H, Kaczmarowski M, Smulski S. Relationship between mastitis agents and somatic cell count in foremilk samples. Bull Vet Inst Pulawy. 2006;50:349–352. [Google Scholar]
- 22.Randolph HE, Erwin RE. Influence of mastitis on properties of milk. X. Fatty acid composition. J dairy sci. 1974;57:865–868. doi: 10.3168/jds.S0022-0302(74)84978-7. https://doi.org/10.3168/jds.S0022-0302(74)84978-7. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence RM, Lawrence RA. Breast milk and infection. Clin Perinatol. 2004;31:501–528. doi: 10.1016/j.clp.2004.03.019. https://doi.org/10.1016/j.clp.2004.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paape M, Weinland B. Effect of abraded intramammary device on milk yield, tissue damage, and cellular composition. J dairy sci. 1988;71:250–256. doi: 10.3168/jds.S0022-0302(88)79549-1. https://doi.org/10.3168/jds.S0022-0302(88)79549-1. [DOI] [PubMed] [Google Scholar]
- 25.Foxman B, D’Arcy H, Gillespie B, Bobo JK, Schwartz K. Lactation mastitis: occurrence and medical management among 946 breastfeeding women in the United States. Am J Epidemiol. 2002;155:103–114. doi: 10.1093/aje/155.2.103. https://doi.org/10.1093/aje/155.2.103. [DOI] [PubMed] [Google Scholar]
- 26.Barbosa-Cesnik C, Schwartz K, Foxman B. Lactation mastitis. JAMA. 2003;289:1609–1612. doi: 10.1001/jama.289.13.1609. https://doi.org/10.1001/jama.289.13.1609. [DOI] [PubMed] [Google Scholar]
- 27.Asadpour R, Tayefi-Nasrabadi H, Moghadam GA, Nofouzi K. Correlation between lactoperoxidase activity and somatic cell count for diagnosis subclinical mastitis in early lactation of dairy cows. J Anim Vet Adv. 2008;7:777–779. [Google Scholar]
- 28.Andrei S, Pintea A, Bunea A, Groza I, Bogdan L, Ciupe S, Matei S, Crainic D. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca Veterinary Medicine. 2009. Non-enzymatic antioxidants concentration and lipids peroxidation level in milk from cows with subclinical mastitis; p. 66. [Google Scholar]
- 29.Patel SH, Vaidya YH, Joshi CG, Kunjadia AP. Culture-dependent assessment of bacterial diversity from human milk with lactational mastitis. Comp Clin Path. 2016;25:437–443. https://doi.org/10.1007/s00580-015-2205-x. [Google Scholar]
- 30.India co. [Acceessed April 2016]. 2011. http://censusindia.gov.in/
- 31.Martín R, Langa S, Reviriego C, Jimínez E, Marín ML, Xaus J, Fernandez L, Rodriguez JM. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr. 2003;143:754–758. doi: 10.1016/j.jpeds.2003.09.028. https://doi.org/10.1016/j.jpeds.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Hunt KM, Foster JA, Forney LJ, Schütte UM, Beck DL, Abdo Z, Fox LK, William JE, McGuire MK, McGuire MA. Characterization of the diversity and temporal stability of bacterial communities in human milk. PloS one. 2011;6:e21313. doi: 10.1371/journal.pone.0021313. https://doi.org/10.1371/journal.pone.0021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiménez E, de Andrés J, Manrique M, Pareja-Tobes P, Tobes R, Martínez-Blanch JF, Codoner FM, Ramon D, Fernandez L, Rodriguez JM. Metagenomic analysis of milk of healthy and mastitis-suffering women. J Hum Lact. 2015;31:406–415. doi: 10.1177/0890334415585078. https://doi.org/10.1177/0890334415585078. [DOI] [PubMed] [Google Scholar]