Abstract
Crustose coralline algae (CCA) fulfill important ecosystem functions in coral reefs, including reef framework stabilization and induction of larval settlement. To investigate in situ the effects of high carbon dioxide on CCA communities, we deployed settlement tiles at three tropical volcanic CO2 seeps in Papua New Guinea along gradients spanning from 8.1 to 7.4 pH. After 5 and 13 months deployment, there was a steep transition from CCA presence to absence around pH 7.8 (660 μatm pCO2): 98% of tiles had CCA at pH > 7.8, whereas only 20% of tiles had CCA at pH ≤ 7.8. As pH declined from 8.0 to 7.8, the least and most sensitive CCA species lost 43% and 85% of cover, respectively. Communities on upward facing surfaces exposed to high light and high grazing pressure showed less steep losses than those on shaded surfaces with low grazing. Direct CO2 effects on early life stages were the main mechanisms determining CCA cover, rather than competitive interactions with other benthic groups. Importantly, declines were steepest at near-ambient pH, suggesting that CCA may have already declined in abundance due to the recent seawater pH decline of 0.1 units, and that future severe losses are likely with increasing ocean acidification.
Crustose coralline algae (CCA, family Corallinaceae, Rhodophyta1) play an important role in many marine ecosystems, providing reef framework, shore protection and carbonate sediments in shallow water2, and facilitating the settlement and survival of larvae of numerous other benthic taxa3,4,5,6. The cell walls of CCA are heavily calcified with high-magnesium calcite7,8, which is more soluble than other forms of calcium carbonate at low pH7. CCA are therefore considered highly vulnerable to ocean acidification (OA), i.e., the rapid global decline in surface seawater pH and associated changes in the seawater carbonate chemistry from rising atmospheric CO2. Several laboratory and mesocosm studies have documented negative effects of OA on CCA calcification, dark dissolution and bleaching9,10, recruitment11, survival12, and rates of morphological abnormalities and mortality in newly settled CCA13. Field studies have also documented severely reduced CCA cover at elevated CO2 at both temperate and tropical volcanic CO2 vents, and other settings with contrasting seawater pH14,15,16,17,18.
Information on differences in the OA susceptibility between individual CCA species is limited, despite their diverse ecological functions, including great inter-specific differences in their effectiveness as reef framework builders19, and as settlement substrata for coral larvae3,4,6. One study showed that in tropical Indo-Pacific CCA, thin encrusting and early successional species, which are particularly important as settlement substrata, appear to be more OA-sensitive than more robust taxa with thicker crusts6. Another study showed that in temperate Northeastern Pacific CCA, two species that form thick crusts have become substantially thinner over the last 20 years, whereas two thinner species maintained similar crust thickness but reduced the thickness of some of their cell walls20. Others have documented an unusually high CO2 tolerance in thick crusts of the tropical Porolithon onkodes, and suggested that this common species will likely continue to provide essential reef and shore protection in future oceans under OA8,21. An improved understanding of the different responses of tropical CCA taxa to OA is therefore warranted, to improve predictions about the future of these communities and the ecosystem services they provide.
To facilitate such predictions, estimates of the in situ response curves of CCA species to increasing CO2 are needed. Biological responses to environmental stressors are often slow and non-linear, hence the choice of stressor concentrations and exposure times in laboratory experiments can strongly influence conclusions about the direction and magnitude of change22,23. Two laboratory studies that investigated the relationships of CCA along pH gradients found linear relationships and no apparent tipping points in CCA growth and survival9,13. However, laboratory experiments are unlikely to reflect ecosystem responses in the field, where a multitude of biotic and environmental factors including light, currents, waves, sedimentation, temperature, recruitment limitation, competition, grazing and acclimatization co-determine CCA abundances19.
There is also conflicting information about the ecological mechanisms that determine how OA will affect CCA. Two experimental studies identified recruitment limitation as the main mechanism of change at near-future levels of OA11,13. In contrast, an in situ study at a temperate rocky shore Mediterranean CO2 vent system concluded that low CCA cover was the outcome from shifts in competitive advantage at reduced pH, while rates of recruitment remained unaltered17.
The objective of this study was to compare the response curves and tolerance thresholds of various CCA taxa to OA, and identify the likely underlying mechanisms for their responses, in situ in coral reefs under both high and low light and grazing pressure. The study was conducted at three volcanic CO2 seeps in Papua New Guinea, which have exposed the reef to additional CO2 for a confirmed 70 years, and possibly much longer15. First, we assessed the cover of CCA in the natural reef communities at the CO2 seeps and at nearby control sites. Second, we investigated the cover of various CCA taxa and of their space competitors on settlement tiles deployed along CO2 gradients for 5 and 13 months. The study was conducted at three CO2 seep sites and three adjacent control sites away from the seeps, at 3 m depth in clear tropical waters. The CCA communities at the seep and control sites were exposed to similar natural conditions of flow, light, waves, sedimentation and temperature, and natural levels of grazing and competitive interactions with other biota. Differences in the communities on the upward and downward facing surfaces of the tiles were used to compare pH responses in high and low light, and under high and low grazing pressure from macro-grazers. The analysis of these data illustrate potential ongoing and future effects of increasing OA on the ecology of common CCA taxa in the tropical Indo-Pacific, and identify potential tipping points in their pH tolerances.
Results
Seawater chemistry
For the reef transects at the three Control sites, the median pH (total scale) ranged from pH 8.02 to 7.98 units and pCO2 ranged from 346 to 413 μatm. At the High CO2 sites, median seawater pH values ranged from 7.95 to 7.72, and calculated pCO2 concentrations ranged from 441 to 998 μatm24.
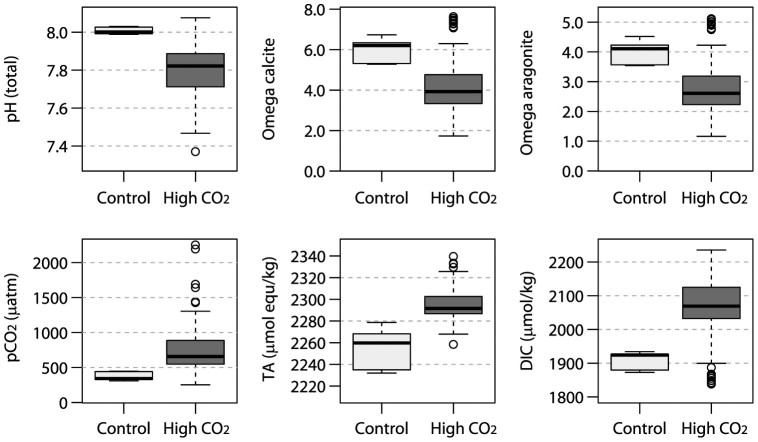
The individual tiles were exposed to seawater chemistry conditions ranging from median values of pH 8.08 to 7.37, 1838 to 2236 μmol kg−1 dissolved inorganic carbon (DIC), and saturation state of calcite (ΩCalc) of 7.6 to 1.73 (Fig. 1, Supplementary Table S1). Differences in the six seawater chemistry parameters between seep and control sites were large and highly significant (Supplementary Table S2). For the tiles from the Control sites, the median pH averaged 8.01 (5th and 95th percentiles: 7.95, 8.09) and the median pCO2 was 397 μatm (300, 484). For the High CO2 tiles, pH averaged 7.80 units (7.52, 7.97), with a median pCO2 concentration of 660 (492, 1128) μatm, total alkalinity (TA) was elevated by ~30 μmol equivalents kg−1 (median: 2292 μmol equivalents kg−1) and dissolved inorganic carbon (DIC) by 140 μmol kg−1 (median: 2069 μmol kg−1). Tiles at the Control sites were exposed to a median Ω Calc of 6.20 (5.29, 7.41), while at the High CO2 sites ΩCalc was 3.93 (2.28, 6.73). Similarly, the median saturation state of aragonite (ΩArag) was reduced from 4.11 (3.55, 4.97) at the Control sites to 2.61 (1.53, 4.52) at the High CO2 sites. Of all tiles, 42% were exposed to a median ΩArag ≤ 3.0, a value commonly assumed a threshold for reef development25. The median ΩArag for all tiles was >1.0, but 10% of the tiles were exposed to Ω Arag ≤ 1.0 for up to 5% of their time.
Figure 1. Seawater chemistry over the Control and High CO2 tiles.
Black horizontal lines indicate medians; boxes enclose the upper and lower quartiles of the data, whiskers mark the maximum and minimum values excluding outliers, while round circles show outliers. The plots show the median values of 45 Control and 71 High CO2 tiles, with each of these 45 and 71 median values composed of 4 to 23 measurements taken directly over each of the tiles during four two-week long visits in the period 2011–2012 (data are listed in the Supplementary Tables S1 and S2, online). pH is at total scale, Omega calcite and aragonite = saturation states of calcite and aragonite, TA = total alkalinity, DIC = dissolved inorganic carbon.
Comparison of CCA between the High CO2 and Control sites
Along the transects on the reef, total CCA cover at the Control sites was ~3-fold higher than at the High CO2 sites, with 6.65% (95% confidence intervals: 4.43%, 8.53%) vs. 2.22% (1.48%, 3.34%); (Fig. 2, SI Table S3). Unoccupied space, i.e. space covered only by biofilms or very sparse turf algae, was ~30% and 24% of natural reef surfaces at the Control and High CO2 sites, respectively.
Figure 2. Log ratios of the cover of crustose coralline algae (CCA) at High CO2 over Control sites, on the three reefs (Reef Communities, N = 80 transects), and on the top- and bottom-sides of the settlement tiles after 5 and 13 months of deployment (5.M, 13.M; N = 120 and 116, respectively).
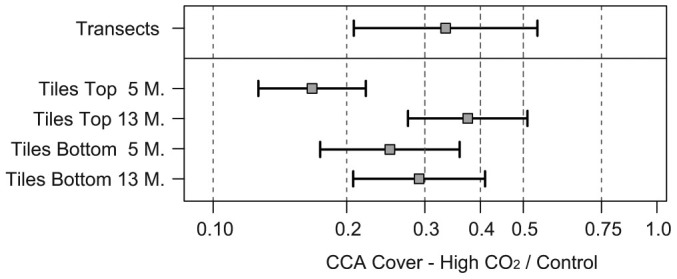
Squares indicate back-transformed means, the error bars are 95% confidence intervals (Supplementary Table S3). For example, the ratio 0.3 indicates the mean cover at High CO2 is 30% of that at the Controls. Differences are all significant at the 5% level (error bars do not include the value 1.0).
On the top-sides of the tiles, total CCA cover also responded strongly to CO2. After 5 months of deployment, total CCA cover was ~6-fold higher on the Control compared to the High CO2 tiles, with cover of 38% (29.7%, 43.3%) vs 6.4% (5.0%, 8.15%); (Figs. 2, 3, Supplementary Table S3). After 13 months, this difference was reduced to 2.7-fold, with 29.6% (23.1%, 35.6%) vs 11% (8.65%, 14.2%), due to a reduction in CCA cover at the Control and slight expansion at the High CO2 sites. Total CCA cover at the 13 month census was significantly correlated to that at the 5 months census (correlation coefficient R2 = 0.50, F(1,63) = 64.6, P < 0.0001). The amount of unoccupied space on the Control tiles declined slightly (from 23% to 17%) between the two censuses, despite slight reductions in CCA and green filamentous algae, due to small expansions of turf algae, macroalgae and cyanobacteria. Unoccupied space on the High CO2 tiles declined substantially, from 36% to 12%, due to the expansion of turf algae, cyanobacteria, CCA and Peysonellia. Most top-sides of the tiles, at both the Control and High CO2 sites, showed dense patterns of recent scrape marks, and macroalgae were restricted to the tile edges, suggesting exposure to intense grazing by macrograzers including fishes and sea urchins (Fig. 3).
Figure 3. CCA settlement tiles at Control sites (left) and High CO2 sites (right) at volcanic CO2 seeps in Papua New Guinea.
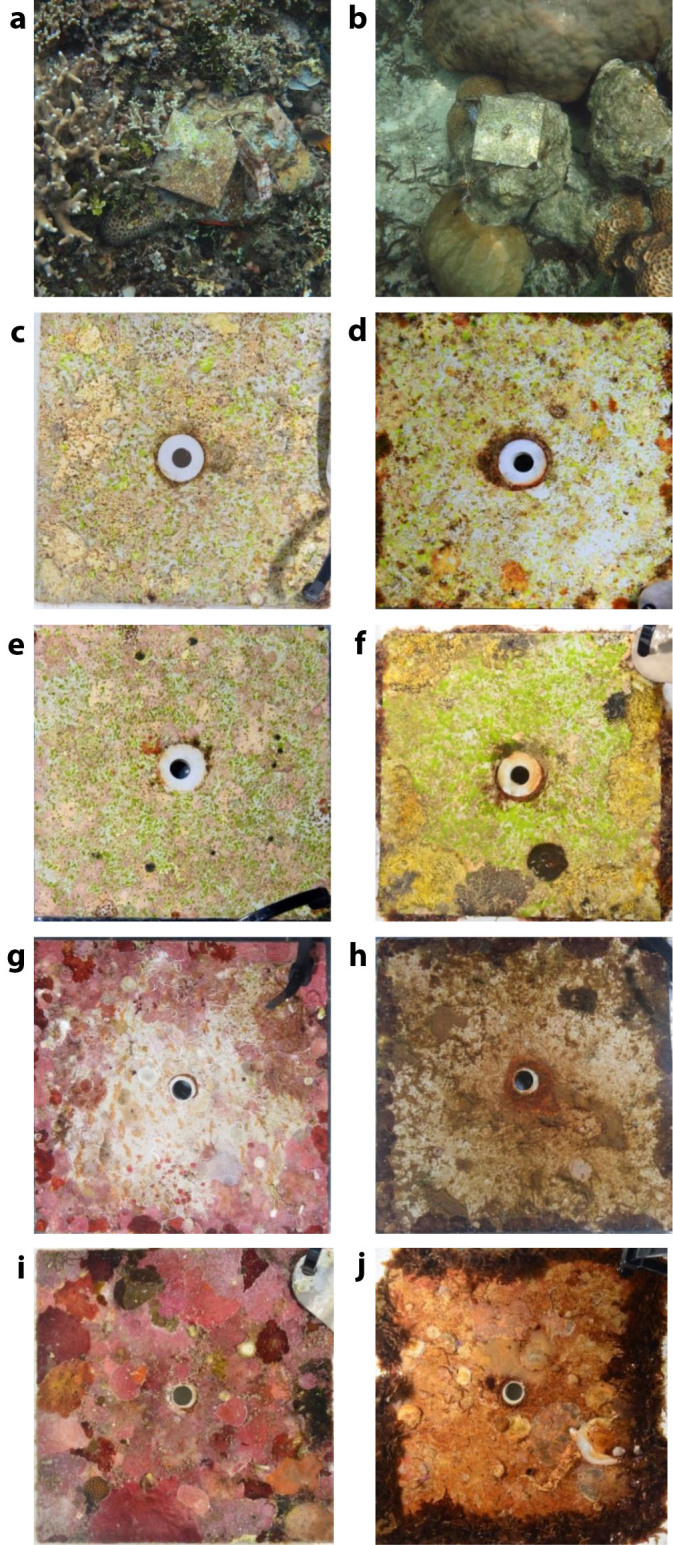
Tiles in situ after 13 months deployment (a, b). Top sides of tiles after 5 months deployment (c, d), and after 13 months (e, f). Bottom sides of the tiles after 5 months (g, h), and after 13 months (i, j).
On the bottom-sides of the tiles, after 5 months of deployment, total CCA cover was ~4-fold higher on the Control compared to the High CO2 tiles, with 23.5% (17.3%, 28.5%) vs 5.9% (4.33%, 7.95%). After 13 months, the difference between Control and High CO2 tiles was 3.4-fold, with 21.4% (16.1%, 25.9%) vs 6.2% (4.69%, 8.26%). As with the top-sides of the tiles, CCA cover on the bottom-sides was significantly correlated between the two census dates (R2 = 0.39, F (1,111) = 69.3, P < 0.0001). Unoccupied space declined between the two censuses, from 21% to 7.6% on the Control tiles, and from 20% to 4.4% at the High CO2 tiles. The reduction in unoccupied space at both Control and High CO2 tiles was due to the expansion of many types of benthic algae and other groups throughout the observation period, while CCA cover remained similar.
Changes in CCA along CO2 gradients
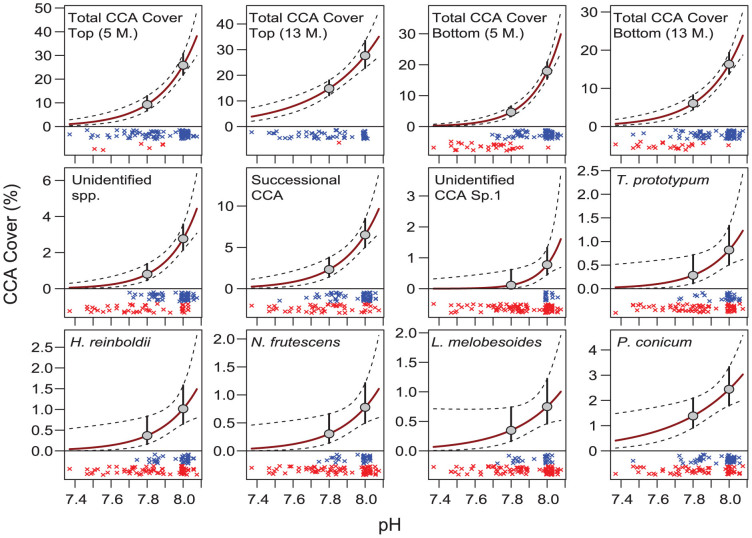
Sorting the tile communities by their median pH levels showed that total CCA cover and the cover of most specific CCA taxonomic groups declined steeply and non-linearly as pH declined from pH 8.1 to 7.4 (Fig. 4). On the top-sides of the tiles, total estimated CCA cover at a median pH of 7.8 (median pCO2 = 660 μatm, ΩArag = 2.76) was 36% and 54% of that at pH 8.0 (pCO2 = 404 μatm, ΩArag = 4.05) after 5 and 13 months, respectively (Supplementary Table S4). Communities were dominated by Porolithon ( = Hydrolithon) onkodes with a >98% contribution to total CCA cover, and hence the estimates of change of total CCA cover on the top-sides largely reflect the response curves of this single species. Its estimated median cover on the 13 months old tiles declined from 27.6% (95% confidence intervals: 22.9%, 33.4%) at pH 8.0 to 14.8% (12.2%, 18.0%) at pH 7.8, and to 4.3% (0.9%, 20%) at pH 7.4. On the bottom-sides of the tiles, CCA declined even more severely, with gradients being much steeper at the high end of the pH range (Fig. 4, Supplementary Table S4). Here, estimated median cover at pH 7.8 was only 26% and 37% of the cover at pH 8.0 after 5 and 13 months, respectively. On the 13 month old tiles, estimated cover was 16.3% (13.8%, 19.3%) at pH 8.0, 6.1% (4.4%, 8.2%) at pH 7.8, and 0.08% (0.02%, 0.40%) at pH 7.4. The species Titanoderma prototypum, Hydrolithon reinboldii, a branching species resembling Neogoniolithon (Spongites) frutescens (taxonomic identification pending), an unidentified species (Unidentified sp.1), and successional CCA were most severely reduced, with 60% to 85% losses of cover at pH 7.8 vs 8.0. The most pH tolerant taxa were Lithoporella melobesioides and Paragoniolithon conicum, which lost 53% and 43% of cover respectively. Estimates for P. onkodes and Lithophyllum kotschyanum from the bottom-sides of the tiles were unreliable due to their rarity on these shaded surfaces (P. onkodes: 6 occurrences, all at pH ≥ 7.79; L. kotschyanum: 5 occurrences, all at pH ≥ 8.0).
Figure 4. Changes in the cover of various taxonomic groups of CCA along the pH gradient (Supplementary Table S4).
Top row: total CCA cover (all CCA taxa combined), on the top- and bottom-sides of the tiles after 5 and 13 months deployment (5 M., 13 M.; N = 120 and 116, respectively). Middle and bottom row: changes in cover of specific CCA taxa on the bottom-sides of the tiles after 13 months. The red solid lines show the estimated cover as a function of pH, dashed lines show upper and lower 95% confidence intervals. The grey dots and vertical bars show mean cover at a pH level of 8.0 and 7.8, and the 95% CI of these estimates. The ‘x’ symbols (jittered vertically for clarity) show the pH of the individual tiles on which the specific CCA taxa were present (blue) or absent (red).
The bottom-sides (low light, little grazing) of the tiles not only had lower CCA cover than the top-sides (high light, intense grazing), but also experienced steeper losses than the top-sides along the pH gradient (Fig. 4, Table 1, Supplementary Table S3). Differences in CCA cover between the two censuses were minor, as the changes along the pH gradient were only marginally weaker after 13 months compared to 5 months.
Table 1. Effects of pH, orientation (top-side with high light and intense grazing, vs bottom side with low light and little grazing), and time (5 vs 13 months of deployment) on CCA cover on the tiles (Fig. 4 - top row). Generalized linear model, and backward elimination of non-significant interaction terms.
| Df | Deviance | F | P | |
|---|---|---|---|---|
| NULL | 264 | 4451.5 | ||
| pH | 1 | 1901.7 | 326.5 | <0.001 |
| Orientation | 1 | 256.8 | 44.10 | <0.001 |
| Time | 1 | 0.600 | 0.103 | 0.748 |
| Tile | 65 | 930.0 | 2.457 | <0.001 |
| pH: Orientation | 1 | 118.1 | 20.28 | <0.001 |
| pH: Time | 1 | 37.84 | 6.498 | 0.012 |
The different responses to CO2 between top- and bottom-sides of the tiles were even more pronounced when using the measure of CCA presence or absence on the tiles. On the top-sides of the tiles, CCA was present on most tiles, even at the lowest pH, with 92% and 96% of tiles having at least some CCA after 5 and 13 months, respectively (Fig. 4). On the bottom-sides of the tiles, CCA was also present on all tiles from the Control sites, and 96% and 98% of tiles at a median pH > 7.8 had at least some CCA after 5 and 13 months, respectively (Figs. 4, 5). However, there was a steep transition from CCA presence to absence at around pH 7.8: at a pH of ≤7.8, only 20% of the tiles had some CCA, and at pH ≤ 7.7, only 3% of tiles had CCA. The proportion of tiles with and without CCA was very similar between the 5 and 13 months censuses (Fig. 5).
Figure 5. Violin plot showing changes in the frequency of settlement tiles with CCA present on their bottom-sides, along the pH gradient.
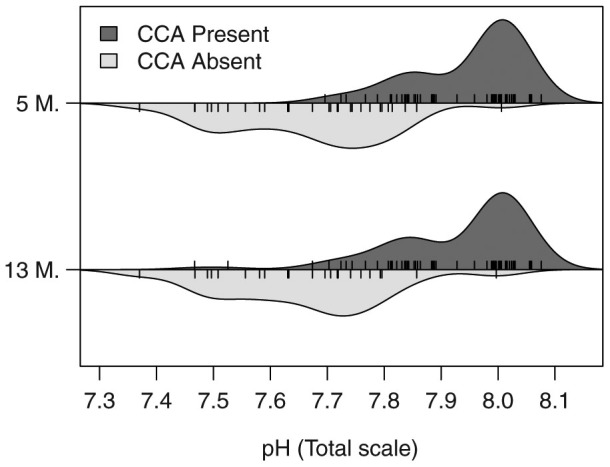
The dark and light grey areas indicate the densities of observations of tiles with and without CCA, respectively, both after 5 month (5 M.: N = 120) and after 13 month of deployment (13 M.: N = 116). The rugs indicate the median pH for each tile, i.e. the pH to which the tiles were exposed to in the field. The transition from CCA presence to absence is steep at a pH of ~7.8.
Discussion
This field study documents strong, non-linear negative relationships between the cover of crustose coralline algae (CCA) and CO2 concentrations on both natural coral reef substrata and on settlement tiles. In shaded conditions, CCA cover was reduced to almost zero at a median pH < 7.7, and none of the CCA taxa were resilient to exposure to high CO2. The most robust and most sensitive of taxa lost 43% and 85% of cover respectively, as the pH declined from 8.0 to 7.8 units. Importantly, the response curves also suggest steep declines between pH 8.1 and 8.0, and thus many CCA species may have already lost cover as a consequence of anthropogenic CO2 emissions, which have lowered the surface seawater pH by 0.1 units compared to pre-industrial times23.
Our results from the colonisation of settlement tiles shed light on the potential underlying mechanisms determining the high CO2 sensitivity of CCA. The amount of unoccupied space on the top-sides of the 5-month old tiles (23% at Control and 36% at High CO2 tiles) was similar to the surrounding reef benthos (24% at Control and 30% at High CO2 sites). CCA cover on the top-sides was 6-fold reduced on the High CO2 compared with the Controls tiles, although the former had 50% more unoccupied space than the latter. On the bottom-sides, the amount of unoccupied space was similar on High CO2 and Control tiles, yet CCA cover was 4-fold reduced. Between the 5 and 13 month censuses, CCA cover gradually increased at High CO2 (6.4% to 11%) but declined at the Control tiles (from 38% to 30%). Thus, in early successional stages, competition by other algal groups appeared to be weaker rather than stronger at elevated CO2, indicating that the reduced CCA cover at High CO2 cannot be attributed to increased competition. As CCA cover at 5 month was well correlated with CCA cover at 13 month, is seems that CO2 directly affected CCA life histories within the first 5 months (recruitment, net growth balancing calcification and dissolution, and/or survival), and that it was these factors that largely determined the later CCA cover.
Our finding of direct effects of CO2 on early CCA life history factors, rather than CO2 effects on later competitive outcomes, contrast with those from settlement tiles deployed at the temperate Mediterranean CO2 seeps17. In the latter, calcareous species including CCA occupied a similar amount of space (<15%) on top-sides of the tiles within the first 3.5 month at both ambient and elevated CO2. After 6.5 and 14 month, CCA cover had not further increased at elevated CO2 due to space occupancy and overgrowth by fleshy macroalgae, while at the control sites CCA cover continued to expand to ~25%, suggesting that shifts in competitive advantages rather than early recruitment determined changes in these CCA communities at elevated CO2. However, our finding of a bottleneck for early CCA life history stages at high CO2 agrees with the results of three other studies. In particular, a 7-week mesocosm study on tropical Indo-Pacific CCA showed a 78% and 92% decline in CCA recruitment and cover respectively, as pH was reduced by 0.26 units from control conditions (an increase in pCO2 from 400 to 765 μatm)11. Similarly, reef-associated CCA species with rapid growth and thin thalli had reduced cover at 400 compared to 800 or 1300 μatm CO2, while CCA taxa with thicker crusts were more CO2 resistant6. In that study, the decline in CCA coincided with higher unoccupied surfaces at elevated CO2, suggesting that direct CO2 effects on the early life history stages, rather than space competition, were responsible for those losses. Young settlers of the cold water CCA species Phymatolithon lenormandii also displayed significantly impaired recruitment success at slightly elevated CO2 within four weeks, due to increased mortality and abnormal development13. This was due to high rates of both dissolution and regrowth over the whole CCA thallus surface at higher CO2, despite similar rates of extension along the growth margins at all pH levels. Finally, Diaz-Pulido et al. (2014) showed that in P. onkodes the mineralogy of deeper skeletons, but not of the actively growing pink surface layers, can change from high magnesium calcite to the less soluble dolomite under OA21. This suggests that such passive change in mineralogy can protect mature established crusts of CCA, but possibly not thin early growth phases. Hence, these studies in combination with ours consolidate the evidence that CO2 predominantly affects early CCA life stages, and that these effects on the early life stages co-determine CCA cover in later successional phases. This conclusion is perhaps not surprising, since CCA sporelings are likely to be particularly sensitive to elevated CO2, with early calcification being essential for the attachment and integrity of the initially very thin films of (<500 μm) hypothallial filaments19. But this finding also suggests that pCO2 perturbation experiments on established CCA crusts may severely underestimate the CO2 effects on future CCA populations, due to the apparent bottleneck of high OA vulnerability in their early life stages.
Grazing intensity may partially explain the different findings about successional mechanisms between the Mediterranean and the PNG seeps study. The former study, which assessed only the top-sides of the tiles in the heavily fished Mediterranean Sea, found that CCA were outcompeted by macroalgae over time17. In our study, the top-sides were heavily scraped indicating exposure to intense grazing that likely prevented macroalgal overgrowth. Two grazing sea urchins (Diadema spp. and Echinometra spp.) are more abundant at the High CO2 sites24, however communities of grazing fishes vary little between the High CO2 and Control sites26. Grazing is considered essential to protect some CCA against overgrowth19, however some taxa have other means to prevent overgrowth, e.g. by regularly sloughing off surface cell layers, and over-intense grazing can also damage CCA. A laboratory study showed that 21 days of exposure to doubled concentrations of CO2 increased the vulnerability of the CCA Hydrolithon ( = Porolithon) onkodes to grazing damage from sea urchins, as CO2 reduced the structural integrity of the CCA cell walls27. On the bottom-sides of our tiles, it is likely that light limitation prevented the overgrowth by turfs or fleshy macroalgae.
Light limitation may further contribute to determining CCA responses to rising CO2. The tiles were deployed at 3 m depth in clear tropical waters, where upward facing surfaces can be exposed to daily irradiances >70% of that at the water surface28. The downward facing sides mimic the light environments in cave entrances and under overhangs, where daily irradiance can be reduced by two orders of magnitude compared to the upward facing reef surfaces28. The declines in CCA cover in response to CO2 were steeper on these shaded bottom-sides compared to the top-sides of the tiles, suggesting that CCA may be physiologically more vulnerable to high CO2 in low compared to high light environments. Previous physiological studies have shown that some CCA can continue to grow at high CO2 in high light environments, but suffer dissolution in the dark29,30. Our data also show the need for high light to maintain populations at high CO2 in the field, with day-time calcification offsetting potential night-time dissolution. Long-term controlled field and mesocosm experiments are needed to better predict the complex interactions between CO2, light and grazing for key tropical CCA species and ecosystems.
Species-specific CO2 tolerances may also be contribute to the different responses, as the top sides of the tiles are dominated by P. onkodes, while the bottom sides had mixed communities. Previous studies have identified mature crusts of P. onkodes as particularly OA tolerant due to the formation of relatively insoluble dolomite in their cell walls8,21. However, we were not able to confirm an unusually high OA tolerance of young P. onkodes on our tiles. On the top-sides P. onkodes lost an estimated 52% of cover as pH declined from 8.0 to 7.8, and on the bottom-sides P. onkodes was only found at a pH ≥ 7.79 pH. On the bottom-sides of the tiles, differences in pH tolerance between species were significant yet relatively minor. Similar to our results, Doropulous et al (2013) identified members of the genera Titanoderma sp. and Hydrolithon spp. as the most CO2 sensitive and P. onkodes the least sensitive CCA taxa, while H. reinboldii appeared more sensitive in our field than in their mesocosm study6. Our observed differences in CO2 sensitivity between species were not attributable to any obvious features. The most sensitive taxa in the field, namely Titanoderma prototypum and Hydrolithon reinboldii, belong to two different subfamilies (subfamily Lithophylloideae and Mastophorideae), while the most pH tolerant taxa (Lithoporella melobesioides, Paragoniolithon conicum and Porolithon onkodes) also belong to the subfamily Mastophorideae. Differences between species were also not attributable to obvious morphological features (e.g., taxa forming thin or thick crusts once mature6,20, or hypothallial structures). However all specimens in our study were still thin crusts (<0.5 mm), probably due to their young age and the heavy grazing regime on the upward facing surfaces.
Our study design facilitated the estimation of CCA response curves to rising CO2 in situ, after many months of exposure. The data show that the declines in cover along the pH gradient were log-linear in both high and low light environments, with steeper losses at the lower compared to the higher end of the CO2 range. There was however no discrete physiological tipping point at any particular level. The data therefore strongly suggest that significant declines in CCA communities are already unfolding in both tropical and temperate waters, and that CCA will continue to decline in the future due to the ongoing rapid increases in CO2.
The greater variability in seawater carbonate chemistry at CO2 seeps compared to future high CO2 oceans31 makes it impossible to use these settings to define exact lower limits for pH tolerance. Recent studies have shown that rates of pH change may contribute to affect the OA vulnerability of CCA, and that acclimatisation to variable pH does not make CCA more tolerant of declining pH32. Nevertheless, as coastal waters can also experience substantial pH variability33, and as exposure to other potential co-limiting factors such as grazing are far more realistic in the field than in the laboratory, lower pH limits are probably best derived from field data, albeit with caution. Furthermore, the CCA on our tiles were exposed to their pH environment since settlement and throughout their life, providing more realistic scope for acclimatisation than short-term laboratory experiments do34. At the PNG seeps, there was a steep transition from tiles with CCA to those without CCA at a pH of ~7.8 (~660 μatm pCO2, ΩArag = 2.8), a value that has been forecasted for the middle of this century by many emissions scenarios. At this level, shaded and light exposed CCA communities were reduced to 37% and 54% of cover at a pH of 8.0. At pH levels of ≤7.7 (~860 μatm pCO2, ΩArag = 2.2), a value that may be reached in the second half of this century, cover was down to ~3% of that at control sites. Similarly, CCA was absent on seagrass blades at ≤7.7 pH at temperate CO2 seeps, suggesting this apparent lowest limit for CCA in situ may not be restricted only to tropical Indo-Pacific CCA communities14. These lower limits match those of the loss in reef development at the PNG seep sites, where reef communities are marginal at 7.8 pH, while at a pH ≤ 7.7, coral reef development ceases with only a few individual coral colonies persisting below this level15. Whether there is a causal relationship between these limits for the CCA communities and for reef development at the PNG seeps remains unknown. However, given the important ecological role of CCA, it is likely that their losses due to anthropogenic CO2 emissions would lead to profound ecological changes in many aspects of benthic marine ecosystems.
Methods
Seawater Chemistry
The study was conducted at three island fringing reefs in Milne Bay Province, Papua New Guinea: Dobu, Esa'Ala and Upa Upasina (latitude 9°45′–9°49′ S, longitude 150°49′–150°52′ E), which are located on an active tectonic fault line where the continental plates of Australia and the Solomon Islands are spreading apart. Each reef contains an area where almost pure (~99%) volcanic CO2 is seeping in shallow water (<5 m depth) from the seafloor, and a control area ~0.5 to 2 km away from each seep with similar geomorphological settings that is not exposed to CO2 seepage15. All sites are very similar in their environmental conditions, including temperature, salinity, light and currents15.
The seawater chemistry data for the reef sites along the transects are published in Fabricius et al. (2014)24. Above each of the numbered tiles, seawater samples were also repeatedly collected during four ~2-week long visits between 2011 and 2013. After returning to the boat, temperature and pH were measured immediately with a pH electrode following standard procedures34. A total of 1134 samples were analysed for pH, with a median of 8 samples per tile (range: 4–23), and a subset were analysed for salinity (Mettler handheld salinity meter). Another subset of 728 samples (median: 5 per tile, range: 3–18) was preserved with mercury chloride in 250 ml polycarbonate bottles for later determination of other seawater carbonate parameters. Of these, 366 samples were analysed for combined total alkalinity (TA) and dissolved inorganic carbon (DIC) with a Vindta 3C (Marianda), the remaining samples were analysed for TA with a Metrohm 855 automated open cell potentiometric titrator35. The remaining seawater carbonate parameters were calculated from the pH, TA, salinity and temperature data with the R program Seacarb v2.4.8 (Lavigne, H. & Gattuso, J. P., http://cran.r-project.org/web/packages/seacarb/index.html).
Of the seawater chemistry variables, the medians and percentiles of pH (converted to total scale), DIC, partial pressure of CO2 (pCO2), and the saturation state of calcite and aragonite (ΩCalc and ΩArag) were all highly correlated (correlation coefficients ranging from 0.81 to 0.97), and median pH was chosen as a proxy for changes in all these variables as predictor variable for the models. TA was less variable and hence more weakly correlated to the other carbonate chemistry variables (correlation coefficients to the other variables: 0.39 to 0.57).
Biotic data
Photo-transects (85 in total, 10 m long, 0.5 m wide, one image every 0.5 m) were used to assess total CCA cover. At Upa-Upasina, 20 and 25 transects were investigated at the High CO2 and Control site, respectively; at the other reefs, the number of transects was 10 per site. Photo analysis followed Jonker et al. (2008), determining the benthos substrata to the highest possible taxonomic resolution underneath 5 fixed points in each image36.
A total of 120 settlement tiles (11.5 × 11.5 × 0.3 cm, made of polyvinyl chloride with surfaces roughened by sand paper) were deployed in December 2011. There was one Control site at each reef, as well as two High CO2 sites at Dobu and Upa-Upasina, and one High CO2 site at Esa'Ala. At each site, 15 tagged tiles were distributed over an area of ~200–400 m2 at 3 m depth. Each tile was secured horizontally ~2 cm above the reef to a tagged base plate. All tiles were first collected after five months (May 2012; all 120 plates were still in place). While being kept submerged in sea water at all times, the top- and bottom-sides were photographed, and tiles were returned to their original location within a few hours of collection. After 13 months deployment (January 2013), the tiles were again collected, with 116 of the tiles still in place. Tiles were again photographed (photographs from 3 of the 8 sites of the 13 months census were lost), rinsed in fresh water, dried, and stored for transport.
To assess benthos cover of the tiles, the images were digitally adjusted for tilt, size, colour and contrast. Grid lines (7 × 7) were overlaid, with the outermost lines crossing 0.5 cm from the tile edges. Substrata were recorded for the 49 grid points, distinguishing 6 and 14 categories for the top- and bottom-sides of the tiles, respectively. Additionally, CCA were identified to highest taxonomic level possible by inspecting conceptacles and other taxonomic features, following Adey et al. (1982)1. For each CCA taxon, the percent cover was visually estimated using a dissecting microscope and a grid as visual guide. Initial inspection showed the top-side communities were dominated (>98%) by the CCA species Porolithon onkodes. On the bottom-sides, eight species, one group of early successional crusts that were too young or too poorly developed to show taxonomic features, and one group of unidentified CCA were distinguished.
Ratios of CCA cover (High CO2/Controls, Fig. 2) were estimated with generalised linear models (GLMs) with log link function and quasipoisson distribution37 and the two predictors pH and reefs. The final analysis included only pH as reef effects were non-significant. GLMs were also used to estimate the effect of pH on the cover of individual species on the tiles, and to predict their cover values for pH conditions at 7.8 and 8.0 (Fig. 4). Tile identity was used as the error term. GLMs were then used to investigate the effects of orientation (top- vs. bottom-sides), and succession (5 vs. 13 months), as well as pH, on total CCA cover (Table 1). All analyses used the statistical package R (R Development Core Team, version 3.0.2), including the R packages Seacarb, beanplot and mgcv.
Supplementary Material
Supplementary Information
Acknowledgments
Special thanks to the families of the Dobu Island, Esa'Ala and Illi Illi Bwa Bwa (Koruwea/Upa Upasina) locations for allowing us to study their reefs, and to Robert Steneck for providing valuable taxonomic advice about the crustose coralline algae. Many thanks also to the crew of the MV Chertan, David Hannan (Plankton Productions), and Roger Steene for supporting many aspects of the field work, and to QantasLink for logistic support. The study was funded by the Australian Institute of Marine Science, and a grant by the National Geographic Society Committee for Research and Exploration.
Footnotes
The authors declare no competing financial interests.
Author Contributions K.E.F. and S.N. designed the research; K.E.F., A.K., L.H., S.N. and G.D. performed the research; G.D. and K.E.F. analyzed the data; all authors contributed to writing the paper.
References
- Adey W. H., Townsend R. A., Boykins W. T. & Press S. I. The Crustose Coralline Algae (Rhodophyta, Corallinaceae) of the Hawaiian Islands. (Smithsonian Institution Press, 1982). [Google Scholar]
- Chisholm J. R. M. Calcification by crustose coralline algae on the northern Great Barrier Reef, Australia. Limnol. Oceanogr. 45, 1476–1484 (2000). [Google Scholar]
- Harrington L., Fabricius K., De'ath G. & Negri A. Recognition and selection of settlement substrata determines post-settlement survival in corals. Ecology 85, 3428–3437, 10.1890/04-0298 (2004). [DOI] [Google Scholar]
- Kitamura M., Koyama T., Nakano Y. & Uemura D. Characterization of a natural inducer of coral larval metamorphosis. J. Exp. Mar. Biol. Ecol. 340, 96–102 (2007). [Google Scholar]
- Price N. Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia 163, 747–758, 10.1007/s00442-010-1578-4 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doropoulos C., Ward S., Diaz-Pulido G., Hoegh-Guldberg O. & Mumby P. J. Ocean acidification reduces coral recruitment by disrupting intimate larval-algal settlement interactions. Ecol. Lett. 15, 338–346, 10.1111/j.1461-0248.2012.01743.x (2012). [DOI] [PubMed] [Google Scholar]
- Andersson A. J., Mackenzie F. T. & Bates N. R. Life on the margin: implications of ocean acidification on Mg-calcite, high latitude and cold-water marine calcifiers. Mar. Ecol.-Progr. Ser. 373, 265–273, 10.3354/meps07639 (2008). [DOI] [Google Scholar]
- Nash M. C. et al. Dolomite-rich coralline algae in reefs resist dissolution in acidified conditions. Nature Clim.Change 3, 268–272, 10.1038/nclimate1760 (2013). [DOI] [Google Scholar]
- Comeau S., Carpenter R. C. & Edmunds P. J. Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate. Proc. Royal Soc. Lond. B-Bio 280, 10.1098/rspb.2012.2374 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noisette F., Egilsdottir H., Davoult D. & Martin S. Physiological responses of three temperate coralline algae from contrasting habitats to near-future ocean acidification. J. Exp. Mar.Biol.Ecol. 448, 179–187, 10.1016/j.jembe.2013.07.006 (2013). [DOI] [Google Scholar]
- Kuffner I. B., Andersson A. J., Jokiel P. L., Rodgers K. S. & Mackenzie F. T. Decreased abundance of crustose coralline algae due to ocean acidification. Nature Geoscience 1, 114–117, 10.1038/ngeo100 (2008). [DOI] [Google Scholar]
- Diaz-Pulido G., Anthony K. R. N., Kline D. I., Dove S. & Hoegh-Guldberg O. Interactions between ocean acidification and warming on the mortality and dissolution of coralline algae. J. Phycol. 48, 32–39 10.1111/j.1529-8817.2011.01084.x (2012). [DOI] [PubMed] [Google Scholar]
- Bradassi F., Cumani F., Bressan G. & Dupont S. Early reproductive stages in the crustose coralline alga Phymatolithon lenormandii are strongly affected by mild ocean acidification. Mar. Biol. 160, 2261–2269, 10.1007/s00227-013-2260-2 (2013). [DOI] [Google Scholar]
- Martin S. et al. Effects of naturally acidified seawater on seagrass calcareous epibionts. Biol. Lett. 4, 689–692, 10.1098/rsbl.2008.0412 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius K. E. et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nature Clim. Change 1, 165–169, 10.1038/nclimate1122 (2011). [DOI] [Google Scholar]
- Price N. N., Martz T. R., Brainard R. E. & Smith J. E. Diel Variability in Seawater pH Relates to Calcification and Benthic Community Structure on Coral Reefs. Plos One 7, 10.1371/journal.pone.0043843 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker K. J., Micheli F. & Gambi M. C. Ocean acidification causes ecosystem shifts via altered competitive interactions. Nature Clim. Change 3, 156–159, 10.1038/nclimate1680 (2013). [DOI] [Google Scholar]
- Porzio L., Garrard S. L. & Buia M. C. The effect of ocean acidification on early algal colonization stages at natural CO2 vents. Mar. Biol. 160, 2247–2259, 10.1007/s00227-013-2251-3 (2013). [DOI] [Google Scholar]
- Steneck R. S. The ecology of coralline algal crusts: convergent patterns and adaptative strategies. Annual Review of Ecology and Systematics 17, 273–303 (1986). [Google Scholar]
- McCoy S. J. & Ragazzola F. Skeletal trade-offs in coralline algae in response to ocean acidification. Nature Clim. Change 4, 719–723, 10.1038/nclimate2273 (2014). [DOI] [Google Scholar]
- Diaz-Pulido G. et al. Greenhouse conditions induce mineralogical changes and dolomite accumulation in coralline algae on tropical reefs. Nat. Commun. 5:3310, 1–9, 10.1038/ncomms4310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries J. B., Cohen A. L. & McCorkle D. C. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134, 10.1130/g30210a.1 (2009). [DOI] [Google Scholar]
- Doney S. C., Fabry V. J., Feely R. A. & Kleypas J. A. Ocean Acidification: The Other CO2 Problem. Ann. Rev. Mar. Sci. 1, 169–192, 10.1146/annurev.marine.010908.163834 (2009). [DOI] [PubMed] [Google Scholar]
- Fabricius K. E., De'ath G., Noonan S. & Uthicke S. Ecological effects of ocean acidification and habitat complexity on reef-associated macro-invertebrate communities. Proc. Royal Soc. Lond. B-Bio 281, 20132479. 10.1098/rspb.2013.2479 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J., Lazar B., Cao L., Caldeira K. & Erez J. Coral reefs may start dissolving when atmospheric CO2 doubles. Geophys. Res. Lett. 36, L05606, 10.1029/2008gl036282 (2009). [DOI] [Google Scholar]
- Munday P. L., Cheal A., Dixson D. L., Rummer J. L. & Fabricius K. E. Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nature Clim. Change 4, 487–492, 10.1038/nclimate2195 (2014). [DOI] [Google Scholar]
- Johnson M. D. & Carpenter R. C. Ocean acidification and warming decrease calcification in the crustose coralline alga Hydrolithon onkodes and increase susceptibility to grazing. J. Exp. Mar. Biol. Ecol. 434, 94–101, 10.1016/j.jembe.2012.08.005 (2012). [DOI] [Google Scholar]
- Anthony K. R. N. & Hoegh-Guldberg O. Variation in coral photosynthesis, respiration and growth characteristics in contrasting light microhabitats: an analogue to plants in forest gaps and understoreys? Funct. Ecol. 17, 246–259 (2003). [Google Scholar]
- Yamamoto S. et al. Threshold of carbonate saturation state determined by CO2 control experiment. Biogeosci. 9, 1441–1450, 10.5194/bg-9-1441-2012 (2012). [DOI] [Google Scholar]
- Martin S., Charnoz A. & Gattuso J.-P. Photosynthesis, respiration and calcification in the Mediterranean crustose coralline alga Lithophyllum cabiochae (Corallinales, Rhodophyta). Eur. J. Phycol. 48, 163–172, 10.1080/09670262.2013.786790 (2013). [DOI] [Google Scholar]
- Kerrison P., Hall-Spencer J. M., Suggett D. J., Hepburn L. J. & Steinke M. Assessment of pH variability at a coastal CO2 vent for ocean acidification studies. Est. Coast. Shelf S. 94, 129–137, 10.1016/j.ecss.2011.05.025 (2011). [DOI] [Google Scholar]
- Kamenos N. A. et al. Coralline algal structure is more sensitive to rate, rather than the magnitude, of ocean acidification. Glob. Change Biol. 19, 3621–3628, 10.1111/gcb.12351 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte C. M. et al. Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries Coasts 36, 221–236, 10.1007/s12237-013-9594-3 (2013). [DOI] [Google Scholar]
- Ragazzola F. et al. Phenotypic plasticity of coralline algae in a High CO2 world. Ecol. Evol. 3, 3436–3446, 10.1002/ece3.723 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangue N. A. et al. A laboratory-based, experimental system for the study of ocean acidification effects on marine invertebrate larvae. Limnol. Oceanogr.-Meth. 8, 441–452, 10.4319/lom.2010.8.441 (2010). [DOI] [Google Scholar]
- Jonker M., Johns K. & Osborne K. Surveys of Benthic Reef Communities Using Underwater Digital Photography and Counts of Juvenile Corals. Vol. 10/2008 (Australian Institute of Marine Science, 2008). [Google Scholar]
- McCullagh P. & Nelder J. A. Generalized Linear Models. (Chapman and Hall, 1989). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information