Figure 3.
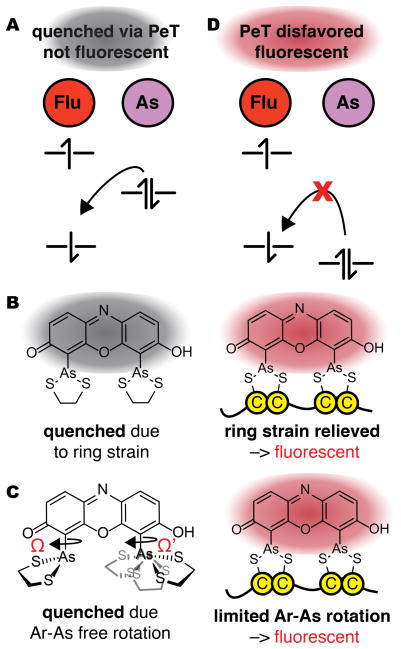
Two mechanisms to account for the increase in ReAsH fluorescence upon binding a tetracysteine (Cys4) motif. (A) In both, ReAsH-EDT2 is quenched by PeT from a high-lying molecular orbital (MO) centered on arsenic to a lower-lying MO centered on the fluorophore. (B) Fluorescence induced by relief of ring strain: In this mechanism, ReAsH-EDT2 fluorescence is quenched by PeT from a As-centered orbital whose energy is raised by strain in the As-EDT chelates; the relief of strain when EDT exchanges for a protein Cys4 motif lowers the energy of this orbital to disfavor PeT and allow fluorescence. (C) In this mechanism, fluorescence induced by restricted rotation: ReAsH-EDT2 fluorescence is quenched by PeT in only some As-aryl bond rotamers; exchange of EDT for a protein Cys4 motif restricts rotation to disfavor PeT and allow fluorescence. (D) In both mechanisms, a change in structure or dynamics lowers the energy of the As-centered orbital to block PeT quenching (Figure 3D).