Abstract
Pathogenesis of coronary artery disease (CAD) is multi-factorial and several conventional risk factors have been ascribed; LDL-C being one of the important risk factor. However Indian population studies with established CAD often show LDL levels within normal range in patients with proven CAD. We hypothesized that Small dense low density lipoprotein (sdLDL) being more atherogenic might correlate more strongly to the occurrence and severity of CAD. The aim of the study was to evaluate the association between serum small dense LDL level and angiographically documented coronary artery disease. This is a cross sectional case control study in which sdLDL were measured in 126 patients with CAD and in 64 patients without CAD. Total cholesterol, HDL Cholesterol, LDL cholesterol and triglycerides were measured by standard methods along with other traditional risk factors. Direct quantitative measurement of sdLDL was done by enzymatic analysis. Mean sdLDL level was higher in patients with coronary stenosis than patients without coronary stenosis (16.3 ± 6.8 vs. 10.1 ± 5.7 mg/dL respectively, (p < 0.001). There was significant correlation between mean sdLDL and severity of CAD as assessed by syntax score with mean sdLDL level in low, intermediate and high syntax score being 15.0 ± 5.8, 20.1 ± 6.7 and 22.7 ± 7.3 mg/dL respectively (p value <0.001). A cut off value of 10.02 mg/dL was associated with presence of CAD (95 % CI 0.82–0.93, p < 0.001) using ROC curve. In conclusion Indian patients with established CAD have higher sdLDL levels compared to individuals without CAD despite having comparable LDL levels.
Keywords: sdLDL, LDL sub fractions, Coronary artery disease, Fasting lipid profile, Lipid metabolism
Introduction
Dyslipidemia has been established as one of the known risk factors for the development of coronary artery disease (CAD) [1, 2]. High levels of plasma low density lipoprotein (LDL-C) concentrations are directly correlated with the development of CAD. However several Indian studies and Western studies on Asian Indians have shown normal LDL-C levels in patients with CAD [3, 4].
LDL-C particles are heterogeneous due to their variations in size, density, and lipid composition [5]. Two evident phenotypes have been recognised using gradient gel electrophoresis viz phenotype A consisting of large buoyant LDL particles with size more than 25.5 nm and phenotype B consisting of small dense LDL (sdLDL) with size 25.5 nm or less [6]. Small dense LDL is considered to be more atherogenic because it readily penetrates the arterial wall and has increased affinity for LDL receptor. It is also more susceptible to oxidation. Several studies have examined the relationship between sdLDL and CAD using gradient gel electrophoresis to determine peak particle size and have found that CAD risk was increased two to threefold in patients with higher sdLDL [7, 8]. Recently, a study evaluated a new automated enzymatic method (DENKA SEIKEN) to determine sdLDL levels and showed association between sdLDL-C and CAD events independent of LDL-C levels [9].
Data pertaining to relationship of LDL particle size and coronary artery disease occurrence is however limited in the Indian population. The aim of the present study was therefore to investigate if the small dense LDL could be a better risk determinant for angiographically proven CAD than traditional total LDL.
Materials and Methods
Subjects
This study was planned as an observational, single centre, cross sectional case control study. Consecutive patients undergoing diagnostic coronary angiography in our institute during period of January 2013 to December 2013 for suspected CAD were prospectively screened. All patients gave informed consent for participation in the study and the study protocol was approved by the institutional ethics committee. The subjects underwent coronary angiography as per clinical need for suspected CAD with one or more of the following indications viz angina like chest pain, shortness of breath resting or exercise ECG abnormality. Patients on statin therapy, acute or chronic renal failure, thyroid disorders, acute infections, stroke, and diabetic ketoacidosis were excluded from the study. The subjects were then divided into two groups depending on the findings on the coronary angiogram:
Positive for CAD (Group 1)—if one or more coronary vessel with diameter stenosis of ≥50 % was detected (Coronary Artery Disease).
Negative for CAD (Group 2 A)—normal coronary arteries with no obstruction detected on coronary angiography (Normal).
Out of 188 screened patients, 38 patients were excluded because of having minimal CAD (stenosis 30–50 %) which fall neither in CAD group nor normal coronary group, underlying renal dysfunction, thyroid disorder, conventional lipid profile not known, triglycerides level >400 mg/dL or inadequate sample collection. 150 patients were included for the final analysis.
Forty healthy volunteers without any history or suspicion for CAD formed the control group (Group 2B). Healthy volunteers were selected from within the staff of the hospital that were otherwise healthy, not a known coronary artery disease and not on any medication for chronic illness. The inclusion criteria for this group were non hypertensive, normal oral glucose tolerance test (OGTT) with normal resting 12 lead ECG and absence of history of angina or myocardial infarction or other risk factors for CAD.
Blood Collection
Blood samples were collected after overnight fasting. Samples were taken in sterile tubes for lipids (total cholesterol, LDL-C, HDL-C, triglyceride) and fasting blood sugar measurement. 4 mL of fasting blood sample was stored at −80 °C for measurement of sdLDL.
Plasma Lipid Analysis
Total cholesterol (TC), HDL-C and triglyceride (TG) were measured by an automated enzymatic technique using a Hitachi 917 autoanalyser from Roche diagnostics. LDL-C was calculated using the Friedewald equation [10]: LDL-C = TC − (TG/5) − HDL-C. Friedewald equation is inaccurate at higher TG levels, therefore subjects with TG of >400 mg/dL were excluded from the LDL analysis.
Method of Estimation of sdLDL
Direct quantitative determination of sdLDL assay was done using LDL-EX “SEIKEN” reagent kits (from Randox laboratories, DENKA SEIKEN UK LTD, United Kingdom). The sdLDL-EX “SEIKEN” is a simple method for the quantitative determination of small dense LDL and consists of two steps viz first step is to filter out large buoyant LDL and other apoB-containing lipoproteins by forming aggregates with the polyanion and divalent cation-based reagent. In the first step, non-sdLDL lipoproteins, that is, chylomicrons, VLDL, IDL, L LDL and HDL are decomposed by a surfactant and sphingomyelinase (SPC) in Reagent-1 that is reactive to those non-sdLDL lipoproteins. The cholesterol released from such non-sdLDL lipoproteins is then degraded to water and oxygen by the action of enzymes. Cholesterol ester is hydrolyzed by the cholesterol esterase (CHE) and then oxidized by the cholesterol oxidase (CO). Produced hydrogen peroxides are finally decomposed to water and oxygen by the catalase.
In the second step, another surfactant in Reagent-2 releases cholesterol only from sd LDL particles and cholesterol released from sdLDL is then subject to the enzymatic reactions. As catalase in the reaction mixture is inhibited by sodium azide in Reagent-2, hydrogen peroxides, produced from the reaction with the cholesterol esterase and cholesterol oxidase, then develop a purple-red colour with the coupler in the presence of peroxidase (POD). For standardisation of the test saline is used as the zero calibrator.
Angiographic Evaluation
Angiograms were analysed by operator blinded to results of lipid analysis. Angiographic CAD was defined as ≥50 % of diameter stenosis in any of the major epicardial coronary arteries [11]. Non CAD group was defined as normal coronaries on angiography. Those with mild disease or irregularities (30–50 % diameter stenosis) were excluded from the analysis. For assessing the severity of the coronary artery disease ‘Syntax score calculator’ was used [12]. The SYNTAX score is an angiographic grading tool to determine the complexity of coronary artery disease. The SYNTAX score is the sum of the points assigned to each individual lesion identified in the coronary tree with >50 % diameter narrowing in vessels >1.5 mm diameter.
Statistical Methods
Assuming a mean sdLDL level of about 30 ± 15 mg/dL in patients without CAD and 40 ± 15 % mg/dL with CAD and 1:2 ratio of inclusion with 95 % power and 5 % level of significance under two tail hypothesis for difference of means we required a minimum numbers of 135 subjects, 45 in No CAD and 90 in CAD group.
Descriptive values are presented as mean ± standard deviation (SD) for continuous variables and percentages for categorical variables. Student t test was used to compare mean values among two groups. Logistic regression analysis was performed to evaluate the association between angiographic CAD and sd-LDL levels. Furthermore, stepwise multivariate logistic regression analysis was performed for correlating several other risk factors for CAD and sdLDL-C levels. All analyses were conducted using SPSS 16 statistical software (SPSS Inc., Chicago, Illinois, USA). A p value <0.05 was considered statistically significant throughout the analysis. ROC curve was established to estimate a cut off value of sdLDL for its association with CAD, if any. For analysis purpose patients with angiographically proven CAD were defined as Group 1 and patients with normal coronaries (Group 2A) and healthy controls (Group 2B) were clubbed into Group 2.
Results
Baseline Characteristics
A total of 188 consecutive patients undergoing coronary angiography were prospectively screened for inclusion in the study and 150 were included in the final analysis. 40 healthy controls were also taken. Depending upon the angiography results patient were divided into two groups as described earlier. Chronic stable angina was present in 68 (54 %) patients, non-ST elevation myocardial infarction (NSTEMI) or unstable angina in 24 (19 %), while recent MI (within last 4 weeks) was present in 34 (27 %) in Group 1. Table 1 shows distribution of lipid levels and other conventional risk factors in the three groups. There was no significant difference in total cholesterol and LDL-C levels in the three groups. Serum triglyceride level was significantly higher and HDL-C level was significantly lower in patients positive for CAD. The mean sdLDL levels in patients positive for CAD and negative for CAD was 16.3 ± 6.8 mg/dL and 10.1 ± 5.7 mg/dL respectively. The sdLDL levels were significantly higher in CAD group (p < 0.001).
Table 1.
Comparison of demographic profile and serum lipid parameters between coronary artery disease patients (Group 1a), normal coronaries (Group 2A) and healthy controls (Group 2B)
| Variables | Positive for CAD (Group 1) n = 126 | Negative for CAD (Group 2A + 2B) Group 2A n = 24 Group 2B n = 40 | Negative for CAD (Group 2; 2A + 2B) n = 64 | Difference between Group 1 and Group 2 (p value) | |
|---|---|---|---|---|---|
| Mean age (years) | 54.6 ± 8.8 | 54.3 ± 6.9 | 48.1 ± 10.1 | 51.2 ± 11.7 | 0.043* |
| Male sex (n/%) | 110 (88 %) | 19 (79 %) | 32 (80 %) | 51 (80 %) | 0.061 |
| BMI (kg/m2) | 25.7 ± 2.4 | 25.8 ± 2.7 | 24.3 ± 3.2 | 24.9 ± 3.1 | 0.057 |
| FBS (mg/dL) | 115.1 ± 50.1 | 109.6 ± 35.7 | 94.9 ± 4.4 | 106.3 ± 32.1 | 0.49 |
| S. creatinine (mg/dL) | 1.1 ± 0.2 | 1.0 ± 0.3 | 0.9 ± 0.1 | 0.9 ± 0.2 | 0.001* |
| DM | 21 (17 %) | 1 (4 %) | 0 (0 %) | 1 (2 %) | 0.002* |
| HTN | 31 (25 %) | 7 (29 %) | 0 (0 %) | 7 (11 %) | 0.022* |
| Smoker | 33 (26 %) | 7 (29 %) | 5 (13 %) | 12 (19 %) | 0.222 |
| Tobacco | 23 (19 %) | 1 (4 %) | 6 (15 %) | 7 (11 %) | 0.164 |
| T cholesterol (mg/dL) | 146.8 ± 30.6 | 156.5 ± 28.9 | 141.4 ± 24.2 | 147.2 ± 26.9 | 0.929 |
| TG (mg/dL) | 183.2 ± 42.9 | 176.2 ± 32.9 | 117.2 ± 49.8 | 139.9 ± 52.5 | <0.001* |
| HDL (mg/dl) | 30.3 ± 8.9 | 33.5 ± 9.5 | 37.7 ± 6.0 | 36.1 ± 7.8 | <0.001* |
| LDL (mg/dL) | 79.5 ± 28.3 | 87.0 ± 27.4 | 80.2 ± 23.7 | 82.8 ± 25.2 | 0.421 |
| sdLDL (mg/dL) | 16.3 ± 6.8 | 12.6 ± 7.1 | 8.5 ± 3.9 | 10.1 ± 5.7 | <0.001* |
BMI body mass index, FBS fasting blood sugar, DM diabetes mellitus, HTN hypertension, TG triglycerides, HDL high density lipoprotein, LDL low density lipoprotein, sdLDL small density lipoprotein
*p < 0.05 is significant
Risk Factors of CAD
A univariate logistic regression analysis between different risk factors and presence of CAD was done (Table 2). The higher age, diabetes mellitus, hypertension, high triglycerides, low HDL and high sdLDL levels were significantly correlated with CAD. Interestingly, LDL levels were not significantly associated with CAD.
Table 2.
Univariate logistic regression analysis of factors for presence of coronary artery disease
| Variable | OR (95 % CI) | p value |
|---|---|---|
| Age | 1.09 (1.06–1.13) | <0.001* |
| BMI | 1.12 (0.99–1.25) | 0.06 |
| Smoking | 1.58 (0.75–3.33) | 0.22 |
| Tobacco chewing | 1.89 (0.76–4.66) | 0.17 |
| DM | 12.9 (4.69–98.4) | 0.013* |
| FBS | 1.0 (0.99–1.01) | 0.35 |
| HTN | 2.73 (1.13–6.61) | 0.03* |
| TC | 1.0 (0.98–1.01) | 0.93 |
| TG | 1.02 (1.01–1.04) | <0.001* |
| HDL | 0.93 (0.89–0.96) | <0.001* |
| LDL | 0.99 (0.98–1.01) | 0.42* |
| sdLDL | 1.25 (1.15–1.35) | <0.001* |
BMI body mass index, FBS fasting blood sugar, DM diabetes mellitus, HTN hypertension, TC total cholesterol, TG triglycerides, HDL high density lipoprotein, LDL low density lipoprotein, sdLDL small density low density lipoprotein
*p < 0.05 is significant
On multivariate logistic regression analysis higher age, high triglyceride and high sdLDL levels emerged as independent predictors for CAD. Diabetes mellitus, hypertension and low HDL levels did not prove to be independent predictors of CAD.
Correlation of sdLDL
A correlation between serum small dense LDL levels and other risk factors was done which showed high sdLDL levels to be associated with increasing age, male sex and higher triglyceride levels which were statistically significant. The mean level of sdLDL was lower in females than males (11.6 ± 5.9 mg/dL vs. 14.8 ± 7.2 mg/dL, p = 0.01). sdLDL levels were positively correlated with age (r = 0.37, p < 0.001), male sex (r = 0.18, p = 0.01), serum triglyceride levels (r = 0.23, p = 0.002). There was negative correlation between sdLDL levels and HDL levels and smoking but was not statistically significant.
sdLDL Levels and severity of CAD
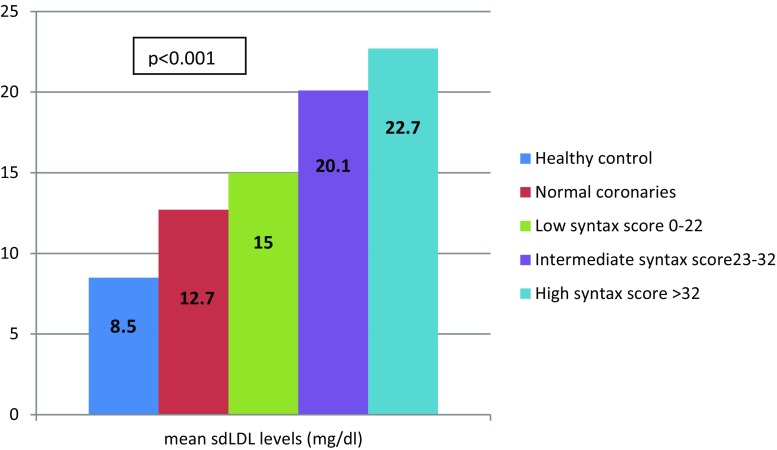
There was a graded and incremental trend observed between mean sdLDL levels and severity of coronary artery disease (Fig. 1). The mean sdLDL was 8.5 ± 3.9 mg/dL in healthy control and 12.7 ± 7.1 mg/dL in patients with risk factors being taken for angiography but found to have normal coronaries as compared to single vessel disease, double vessel disease and triple vessel disease which was 14.3 ± 5.8, 16.1 ± 6.7 and 18.2 ± 7.3 mg/dL respectively with p value <0.0001. For assessing the severity of the CAD groups were further divided into 3 groups on the basis of syntax score. There was linear relationship between sdLDL levels and the syntax score. The mean sdLDL was 15.0 ± 6.1 mg/dL in low syntax score, 20.1 ± 8.0 mg/dL in intermediate syntax score and 22.7 ± 3.6 mg/dL in high syntax score. The trends suggested a linear relation between mean sdLDL levels and extend of disease.
Fig. 1.
Graded and incremental levels of mean sdLDL according to severity of coronary artery disease as assessed by syntax score
Cut off value of sdLDL analysis by receiving operating curve (ROC) analysis (Fig. 2) revealed sdLDL level >10.02 mg/dL to be predictive of CAD with 82 % sensitivity and 83 % specificity. The area under the curve (AUC) was relatively high (AUC = 0.83, 95 % CI = 0.82–0.93, p < 0.0001) suggestive of high sensitivity and specificity to predict association of sdLDL with CAD.
Fig. 2.
Receiver operating characteristics (ROC) curve for predicting presence of coronary artery disease, using data from the case-control cross-sectional study described
Discussion
Results of our study showed sdLDL as an independent risk factor for CAD. It showed patients with CAD had increased levels of triglyceride and reduced levels of HDL-C. There was no significant difference between LDL-C among three groups. sdLDL was significantly higher in patients with CAD. It has been reported that Asian Indians have a typical dyslipidemia characterized by high triglycerides and low HDL levels with near normal LDL cholesterol levels [3, 4] when compared to western population with high levels of LDL. Our patients in this study also had LDL levels largely within in normal range.
A few earlier studies [13, 14] compared small dense LDL distribution in migrant Indians and Europeans, but they showed contradictory results with one showing a higher frequency of small dense LDL in Indians [13] while the other reporting Indians to have larger LDL size [14]. However, both these studies merely looked at the frequency of LDL sub fractions in Indians and not specifically to its association with CAD.
Atherogenicity of sdLDL is due to its high oxidizability owing to low cholesterol and high PUFA and ApoB content [5]. This results in the reduction of LDL clearance by its receptors, with increased production of scavengers which triggers immunological changes resulting in atherosclerosis. Apart from increased atherogenesis, increased levels of sdLDL are associated with elevated fibrinogen and plasminogen activator inhibitor protein-1 (PAI-1) concentrations both of which are thrombogenic leading to increase event rates [15].
There was significant positive correlation between sdLDL with male sex, increasing age, triglyceride levels and a significant negative correlation with HDL levels. Females are expected to have lower levels of sdLDL due to lower levels of hepatic lipase activity in females [16] with our results being consistent. Earlier studies have also shown a positive association of triglyceride levels with sdLDL [17, 18]. The key abnormality leading to the generation of sdLDL is the development of mild to moderate hypertriglyceridemia [17].
The mean sdLDL level was significantly higher in CAD patients as compared to those without CAD (16.3 ± 6.8 vs. 10.1 ± 5.7 mg/dL, p < 0.001). These values are consistent with earlier study done in Indian population. CURES-8 showed mean sdLDL in patients without CAD to be 7.2 ± 6.8 mg/dL as compared to CAD patients with mean sdLDL of 16.7 ± 11.1 mg/dL [19].
The effect of statin on sdLDL concentration is controversial. Statins do not effect particle composition but they do strongly effect the LDL concentration and the particle number. Statin therapy to lower overall LDL concentration is felt to be a very powerful intervention in terms of lowering atherosclerosis risks and preventing atherosclerotic events. Choi et al. [20] demonstrated that statin therapy reduces total LDL-C, absolute amounts of small, dense LDL, and absolute amounts of large, buoyant LDL but the proportion of sdLDL to total LDL is rather increased. Yoshino et al. [21] showed that rosuvastatin reduced plasma sdLDL and larger LDL, though the effect was markedly greater with the former. However, this effect was significant only in non-diabetic hypercholesterolemic patients.
We also tried to correlate sdLDL-cholesterol levels to the severity of coronary artery disease. The mean sdLDL-cholesterol level increased with the number of coronary arteries affected and the syntax score [12]. It is similar to study by Toft-Petersen et al. [22]; a case control study of 194 patients, which found that coronary stenosis was highly correlated with proportion of sdLDL-cholesterol.
Limitations of our study include firstly a small cohort of patients. Secondly, the possibility of occult coronary artery disease cannot be ruled out in healthy volunteers even though probability of CAD would be rather low, since they were clinically asymptomatic. Lastly, the LDL cholesterol was calculated from Friedewald’s equation and was not directly measured.
In conclusion, our findings showed that Indian patients with established CAD have higher sdLDL levels compared to healthy individuals despite having comparable LDL levels. sdLDL is an independent risk factor for CAD and levels >10.02 mg/dL are associated with significant CAD. This being small study further studies are needed to evaluate the cut off level for sdLDL. It is suggested to look beyond traditional lipid profile and estimate sdLDL which may assist in identifying a higher risk population especially in absence of other known risk factors.
Compliance with Ethical Standards
Conflict of interest
There is no conflict of interests.
Contributor Information
Pravin K. Goel, Email: golf_pgi@yahoo.co.in
Fauzia Ashfaq, Email: farooqui23@yahoo.co.in.
Roopali Khanna, Phone: 00915222494223, Email: drroopalik@gmail.com.
V. Ramesh, Email: vramesh@sgpgi.ac.in
Chandra Mohan Pandey, Email: cmpandey@sgpgi.ac.in.
References
- 1.Smith SC, Jackson R, Pearson TA, Fuster V, Yusuf S, Faergeman O, et al. Principles for national and regional guidelines on cardiovascular disease prevention: scientific statement from the world heart and stroke forum. Circulation. 2004;109:3112–3121. doi: 10.1161/01.CIR.0000133427.35111.67. [DOI] [PubMed] [Google Scholar]
- 2.Penalva RA, Huoya Mde O, Correia LC, Feitosa GS, Ladeia AM. Lipid profile and intensity of atherosclerosis disease in acute coronary syndrome. Arq Bras Cardiol. 2008;90(1):24–30. doi: 10.1590/S0066-782X2008000100005. [DOI] [PubMed] [Google Scholar]
- 3.Goel PK, Bharti BB, Pandey CM, Singh U, Tewari S, Kapoor A, et al. A tertiary care hospital-based study of conventional risk factors including lipid profile in proven coronary artery disease. Indian Heart J. 2003;55:234–240. [PubMed] [Google Scholar]
- 4.Mohan V, Deepa R, Rani SS, Premalatha G. Prevalence of coronary artery disease and its relationship to lipids in a selected population in South India: the Chennai Urban Population Study (CUPS No. 5) J Am Coll Cardiol. 2001;38(3):682–687. doi: 10.1016/S0735-1097(01)01415-2. [DOI] [PubMed] [Google Scholar]
- 5.Krauss RM. Heterogeneity of plasma low density lipoproteins and atherosclerotic risk. Curr Opin Lipidol. 1994;5:339–349. doi: 10.1097/00041433-199410000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Campus H. Low density lipoprotein size and cardiovascular disease: a reappraisal. J Clin Endocrinol Metab. 2003;88:4525–4532. doi: 10.1210/jc.2003-030636. [DOI] [PubMed] [Google Scholar]
- 7.Koba S, Hirano T, Yoshino G, Sakai K, Sakaue T, Adachi M, Katagiri T. Remarkably high prevalence of small dense low density lipoprotein in Japanese men with coronary artery disease. Atherosclerosis. 2002;160:249–256. doi: 10.1016/S0021-9150(01)00580-9. [DOI] [PubMed] [Google Scholar]
- 8.Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, et al. Small dense low density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec cardiovascular Study. Circulation. 1997;95:69–75. doi: 10.1161/01.CIR.95.1.69. [DOI] [PubMed] [Google Scholar]
- 9.Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, Hoefner DM, Remaley AT. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:196–201. doi: 10.1161/ATVBAHA.113.302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin SS, Blaha MJ, Elshazly MB, Brinton EA, Toth PP, McEvoy JW, et al. Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. J Am Coll Cardiol. 2013;62(8):732–739. doi: 10.1016/j.jacc.2013.01.079. [DOI] [PubMed] [Google Scholar]
- 11.Meijboom WB, Van Mieghem CA, van Pelt N, Weustink A, Pugliese F, Mollet NR, et al. Comprehensive assessment of coronary artery stenoses computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. 2008;52:636–643. doi: 10.1016/j.jacc.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Serruys PW, Morice MC, Kappetein AP, Antonio Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni KR, Markovitz JH, Nanda NC, Segrest JP. Increased prevalence of smaller and denser LDL particles in Asian Indians. Arterioscler Thromb Vasc Biol. 1999;19:2749–2755. doi: 10.1161/01.ATV.19.11.2749. [DOI] [PubMed] [Google Scholar]
- 14.Abate N, Garg A, Enas EA. Physico-chemical properties of low-density lipoproteins in normolipidemic Asian Indian men. Horm Metab Res. 1995;27:326–331. doi: 10.1055/s-2007-979971. [DOI] [PubMed] [Google Scholar]
- 15.Väisänen S, Baumstark MW, Penttilä I, Bouchard C, Halonen P, Rankinen T, Berg A, Rauramaa R. Small, dense LDL particle concentration correlates with plasminogen activator inhibitor type-1 (PAI-1) activity. Thromb Haemost. 1997;78(6):1495–1499. [PubMed] [Google Scholar]
- 16.Gohari LH, Ghassab RK, Firoozray M, Zavarehee A, Basiri HA. The association between small dense low density lipoprotein, apolipoprotein B, apolipoprotein B/apolipoprotein A1 ratio and coronary and coronary artery stenosis. Med J Islam Repub Iran. 2009;23(1):8–13. [Google Scholar]
- 17.Griffin BA, Freeman DJ, Tait GW, Thomson J, Caslake MJ, Packard CJ, et al. Role of plasma triglyceride in the regulation of plasma low density lipoprotein subfractions: relative contribution of small, dense LDL to coronary heart disease risk. Atherosclerosis. 1994;106:241–253. doi: 10.1016/0021-9150(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 18.Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, Ballantyne CM. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 2014;34(5):1069–1077. doi: 10.1161/ATVBAHA.114.303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohan V, Deepa R, Velmurugan K, Gokulakrishnan K. Association of small dense LDL with coronary artery disease and diabetes in urban Asian Indians—the Chennai urban rural epidemiology study (CURES-8) J Assoc Physicians India. 2005;53:95–100. [PubMed] [Google Scholar]
- 20.Choi CU, Seo HS, Lee EM, Shin SY, Choi UJ, Na JO, et al. Statins do not decrease small, dense low-density lipoprotein. Tex Heart Inst J. 2010;37(4):421–428. [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshino G, Nakano S, Matsumoto T, Murakami E, Morita T. Rosuvastatin reduces plasma small dense LDL-cholesterol predominantly in non-diabetic hypercholesterolemic patients. Pharmacol Pharm. 2012;3:72–78. doi: 10.4236/pp.2012.31011. [DOI] [Google Scholar]
- 22.Toft-Petersen AP, Tilsted HH, Aarøe J, Rasmuseen K, Christensen T, Griffin BA, et al. Small dense LDL particles—a predictor of coronary artery disease evaluated by invasive and CT-based techniques: a case-control study. Lipids Health Dis. 2011;10:21. doi: 10.1186/1476-511X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]