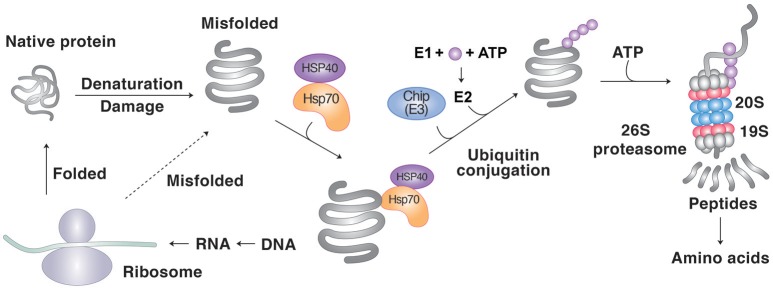
Figure 1.
Protein degradation by molecular chaperones through the UPS. Molecular chaperones such as Hsp70 recognizes the hydrophobic sequences of misfolded proteins as degrons. The Ub ligase CHIP guides the chaperone-client complexes to the UPS and mediates the clients' ubiquitination. The UPS involves a cascade of E1, E2, and E3 enzymes whose cooperative activities mediate the conjugation of Ub to target proteins. In PQC, most E3s cannot recognize misfolded proteins and rather depend on molecular chaperones for substrate recognition. Ubiquitinated substrates are degraded by the proteasome into short peptides, typically with sizes of 8–12 amino acids.