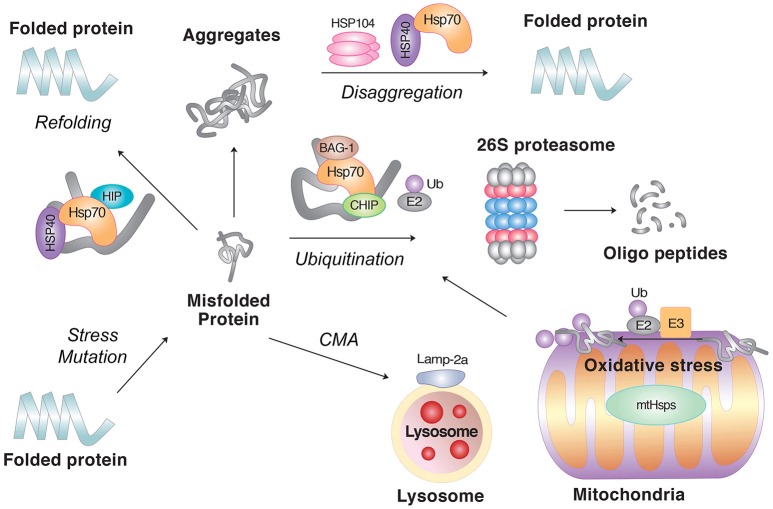
Figure 2.
The role of molecular chaperones in PQC. Molecular chaperones, such as Hsp70 in combination with the cochaperone Hsp40, facilitate the refolding of misfolded proteins. If the clients fail to refold, molecular chaperones can also mediate their degradation in collaboration with cellular proteolytic pathways. In principle, soluble misfolded proteins are targeted by the UPS, in which the clients are ubiquitinated by E3 Ub ligases followed by degradation through the 26S proteasome. However, if the clients are prone to aggregation or escape the surveillance of the UPS, they can be degraded by lysosomal hydrolases, either through macroautophagy or CMA. As the last step of PQC, molecular chaperones can disaggregate already formed aggregates. Also shown are misfolded proteins induced by oxidative stress in mitochondria.