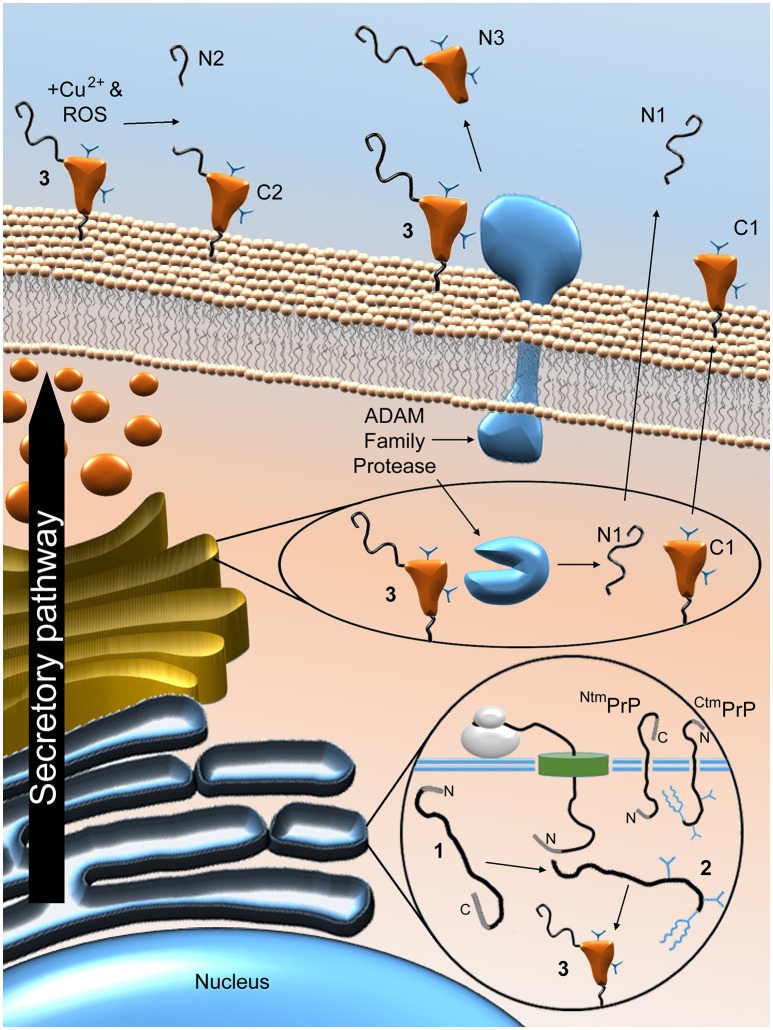
Figure 4.
Proteolytic processing of PrPC. Post-translational and proteolytic processing events create multiple distinct PrP fragments. Ribosomal expression of PrPC is concomitant with ER translocation. Imperfect translocation can result in NtmPrP or CtmPrP. Once in the ER, the immature protein (1) is N- and C-terminally truncated, glycosylated, the membrane anchor is added and the single disulphide bond is formed to produce the mature protein (2), before (potentially chaperone-mediated) folding to produce the folded form (3). Enzymatic α-cleavage, possibly mediated by ADAM family proteases, results in the production of N1 and C1 and is thought to occur either in an acidic endosomal compartment or within the Golgi apparatus. These fragments and the remaining, uncleaved PrPC molecules are trafficked to the cell surface. Once there, PrPC can be subject to β-cleavage, possibly stimulated by the combined presence of ROS and Cu2+, leading to the production of N2 and C2. ADAM protease-mediated shedding may also occur, which results in cleavage of PrPC near its GPI anchor, thereby producing the N3 fragment. The sites of proteolytic cleavage are shown schematically in Figure 2B.