Abstract
Campylobacter is one of the major foodborne pathogens that result in severe gastroenteritis in humans, primarily through consumption of contaminated poultry products. Chickens are the reservoir host of Campylobacter, where the pathogen colonizes the ceca, thereby leading to contamination of carcass during slaughter. A reduction in cecal colonization by Campylobacter would directly translate into reduced product contamination and risk of human infections. With increasing consumer demand for antibiotic free chickens, significant research is being conducted to discover natural, safe and economical antimicrobials that can effectively control Campylobacter colonization in birds. This study investigated the efficacy of in-feed supplementation of a phytophenolic compound, β-resorcylic acid (BR) for reducing Campylobacter colonization in broiler chickens. In two separate, replicate trials, day-old-chicks (Cobb500; n = 10 birds/treatment) were fed with BR (0, 0.25, 0.5, or 1%) in feed for a period of 14 days (n = 40/trial). Birds were challenged with a four-strain mixture of Campylobacter jejuni (∼106 CFU/ml; 250 μl/bird) on day 7 and cecal samples were collected on day 14 for enumerating surviving Campylobacter in cecal contents. In addition, the effect of BR on the critical colonization factors of Campylobacter (motility, epithelial cell attachment) was studied using phenotypic assay, cell culture, and real-time quantitative PCR. Supplementation of BR in poultry feed for 14 days at 0.5 and 1% reduced Campylobacter populations in cecal contents by ∼2.5 and 1.7 Log CFU/g, respectively (P < 0.05). No significant differences in feed intake and body weight gain were observed between control and treatment birds fed the compound (P > 0.05). Follow up mechanistic analysis revealed that sub-inhibitory concentration of BR significantly reduced Campylobacter motility, attachment to and invasion of Caco-2 cells. In addition, the expression of C. jejuni genes coding for motility (motA, motB, fliA) and attachment (jlpA, ciaB) was down-regulated as compared to controls (P < 0.05). These results suggest that BR could potentially be used as a feed additive to reduce Campylobacter colonization in broilers.
Keywords: Campylobacter jejuni, β-resorcylic acid, pre-harvest safety, chickens, colonization factors, gene expression, cell culture
Introduction
Campylobacter contamination of food products is the leading cause of bacterial foodborne illness worldwide (Crim et al., 2015; Mangen et al., 2016). Campylobacter, in particular, Campylobacter jejuni, is the second most commonly reported foodborne pathogen in the USA with an annual incidence of 13.45 per 100,000 resulting in approximately 1.3 million infections annually (Crim et al., 2015). Actual cases are probably higher than these estimates due to under-reporting or sick individuals not seeking medical attention (Mead et al., 1999; Samuel et al., 2004). Campylobacter causes mild to severe gastroenteritis, fever, vomiting, and diarrhea in patients. In a minority of cases, it leads to more serious Guillain–Barré syndrome, reactive arthritis, or irritable bowel syndrome (Spiller, 2007; Gradel et al., 2009). Epidemiological studies have shown that the major risk factors associated with Campylobacter infections are improper handling and consumption of chicken or other food products cross-contaminated with poultry meat or juice during food preparation (Rosenquist et al., 2003; Friedman et al., 2004; Danis et al., 2009). The low infectious dose of C. jejuni (∼500 CFU) further raises public health concerns since only a few microorganisms are needed to cause the infection (Black et al., 1988).
Campylobacter sp. colonizes the gastrointestinal tract of chickens by the third to fourth week of age as a commensal organism (Annan-Prah and Janc, 1988; Stern et al., 1988; Humphery et al., 1993; Dhillon et al., 2006). Various studies have reported enteric colonization up to 108 CFU/g of cecal contents in birds (Beery et al., 1988; Achen et al., 1998). Product contamination mostly occurs during slaughtering of chickens (Berrang et al., 2000; Herman et al., 2003; Reich et al., 2008; Boysen et al., 2016). Therefore, effective strategies to control Campylobacter in poultry flocks at the farm level are needed to reduce product contamination and the incidence of campylobacteriosis in humans (Rosenquist et al., 2003; Arsenault et al., 2007; Reich et al., 2008).
A variety of pre-harvest strategies have been employed to reduce Campylobacter in poultry with varied degree of success. These include feeding birds with bacteriophages (Carrillo et al., 2005; Wagenaar et al., 2005), bacteriocins (Stern et al., 2005; Svetoch and Stern, 2010), probiotics (Santini et al., 2010; Arsi et al., 2015; Shrestha et al., 2017), and vaccination (Buckley et al., 2010; Chintoan-Uta et al., 2016). With increasing consumer demand for safe and natural products with minimal preservatives, significant research is being conducted to explore the potential of natural antimicrobials for controlling C. jejuni in chickens (Hermans et al., 2011b).
Since ancient times, plant compounds have been used for improving shelf life and microbiological safety of food. The antimicrobial activity of several phytochemicals has been previously reported (Burt, 2004; Holley and Patel, 2005; Upadhyay et al., 2014). β-resorcylic acid (BR; 2, 4 dihydroxybenzoic acid) is a phytophenolic compound that is widely distributed among the angiosperms as a secondary metabolite to protect plants against pathogens (Friedman et al., 2003). It is classified under “Everything Added to Food in the United States” (EAFUS; Cas no. 89-86-1) by the US-FDA (U. S. FDA EAF 3045; U. S. Food and Drug Administration, 2013). Previous research has shown that BR is effective in reducing major foodborne pathogens, including Salmonella (Mattson et al., 2011), Listeria monocytogenes (Upadhyay et al., 2013), and Escherichia coli O157:H7 (Baskaran et al., 2013) in food products. However, its efficacy in reducing C. jejuni in chickens has not been determined.
The objective of this study was to investigate the efficacy of in-feed supplementation of BR in reducing C. jejuni colonization in broiler chickens. In addition, the effect of BR on the various virulence factors critical for Campylobacter colonization in chickens was investigated.
Materials and Methods
Campylobacter Strains and Culture Conditions
Four wild strains (S-1, S-3, S-4, S-8) of C. jejuni originally isolated from the commercial broilers were used in the study. Each strain was grown separately in 10 ml of Campylobacter enrichment broth (CEB, International Diagnostics Group, Bury, Lancashire, UK) for 48 h at 42°C under microaerophilic condition (5% O2, 10% CO2, and 85% N2).
Anti-Campylobacter Efficacy of BR in Chicken Cecal Contents
The antibacterial activity of BR against C. jejuni in cecal contents was investigated as described before (Kollanoor Johny et al., 2010). Cecal contents from broiler chickens were collected and autoclaved at 121°C for 15 min. The autoclaved cecal contents (10 ml) were inoculated with C. jejuni to ∼108 CFU/ml. Different concentrations of treatment solution were prepared by dissolving appropriate quantities of BR (Sigma-Aldrich, Co., St. Louis, MO, USA) in CEB to make a final concentration of 0.25, 0.50, and 1% BR. Campylobacter enrichment broth was used as a control. Then, 100 μl of cecal content inoculated with the four-strain mixture of C. jejuni and 900 μl of respective treatment solutions were added in tubes and incubated at 42°C under microaerophilic condition for 24 h. Duplicate samples were serially diluted (1:10) in Butterfield’s phosphate diluent (BPD, 0.625 mM potassium dihydrogen phosphate, pH 7.2) and plated at 0, 8, 24 h on Campylobacter line agar (CLA; Line, 2001), followed by incubation at 42°C for 48 h to enumerate the surviving C. jejuni.
Bird Housing, Campylobacter Challenge, and Enumeration
Two in vivo studies were conducted with a total of 80 birds. In each trial, 40 day of hatch broiler chicks (Cobb500) were obtained from a commercial hatchery. All the experiments were approved by the Institutional Animal Care and Use Committee of the University of Arkansas and recommended guidelines were followed for animal handling. Birds were weighed and randomly allocated to one of four treatments groups (0, 0.25, 0.5, 1% BR) (n = 10 chicks/treatment/trial). BR was thoroughly mixed in mash feed using a commercial feed mixer and was fed throughout the 14-day study period along with ad libitum water. The feed consumption, initial and final body weights of individual birds was recorded during the study. Birds were challenged via oral gavage with a cocktail of four wild strains of C. jejuni (6 Log CFU/ml; 250 μl/bird) on day 7. On day 14, cecal contents were collected aseptically, serially diluted and plated on CLA for enumeration of Campylobacter (Cole et al., 2006). Confirmation of Campylobacter colonies was made with a latex agglutination test (Scimedx, Co., Dover, NJ, USA).
Determination of SIC of BR
The sub-inhibitory concentration (SIC) of BR was determined as described previously (Upadhyay et al., 2012). Briefly, two-fold dilutions of BR (0, 0.1, 0.05, 0.025, 0.0125, 0.00625, and 0.003125%) were made in a sterile 24-well polystyrene plate (Costar, Corning, NY, USA) containing CEB followed by inoculation with wild type S-8 strain of C. jejuni (∼106 CFU/ml). The plates were incubated at 42°C under microaerophilic condition for 24 h and the growth of C. jejuni was enumerated. The highest concentration of BR that did not inhibit the growth of C. jejuni as compared to controls was selected as the SIC of the compound.
Motility Assay
The effect of BR on the motility of C. jejuni was determined as described previously (Upadhyay et al., 2012). Motility test agar plates (Becton, Dickinson and Company, Sparks, MD, USA) with or without (control) SIC of BR were prepared and stab inoculated in the center with 5 μl culture of C. jejuni strain S-8 (6 log CFU). The plates were incubated for 48 h at 42°C under microaerophilic condition and the zone of motility were measured.
Adhesion and Invasion Assays
The effect of SIC of BR on C. jejuni adhesion to and invasion of human Caco-2 cells was investigated as described previously (Moroni et al., 2006) with modifications. Human enterocytes were maintained in DMEM (VWR Life Science, Rochester, NY, USA) containing 10% fetal bovine serum (VWR Life Science, Rochester, NY, USA). Caco-2 cells were grown in sterile 24-wells culture plates (∼105 cells per well) to form monolayer at 37°C in a humidified, 5% CO2 incubator. The Caco-2 monolayer was inoculated with the mid-log phase of C. jejuni S-8 (∼6 Log CFU/ml; multiplicity of infection 10:1) either in the presence or absence of SIC of BR. For the adhesion assay, infected monolayer (after an hour of incubation at 42°C under microaerophilic condition) was rinsed twice in minimal media and lysed with 0.1% Triton-X 100 (Sigma-Aldrich, Co., St. Louis, MO, USA) for 15 min. The enumeration of adherent C. jejuni S-8 was made by serial dilution and plating on CLA. For the invasion assay, infected cells (after an hour of incubation) were rinsed twice in minimal media followed by 2 h of incubation in whole media containing gentamicin (200 μg/ml) (Sigma-Aldrich, Co., St. Louis, MO, USA) to kill extracellular bacteria. After incubation, the cells were processed for enumerating the number of invaded bacteria as described above.
RNA Isolation, cDNA Synthesis, and Real-Time Quantitative PCR
The effect of SIC of BR on the expression of Campylobacter chicken colonization genes was studied using real-time quantitative PCR (RT-qPCR) as described previously (Woodall et al., 2005; Upadhyay et al., 2012). C. jejuni strain S-8 was cultured with or without SIC of BR in CEB at 42° C under microaerophilic condition and total RNA was extracted at mid-log phase (10 h) using RNA mini kit (Invitrogen, Carlsbad, CA, USA). The complementary DNA (cDNA) was made using iScript cDNA synthesis kit (Bio-Rad) after DNase treatment (Thermo Fisher Scientific, Carlsbad, CA, USA). All the primers in our study (Table 1) were designed from published Gene Bank C. jejuni sequences using Primer 3 software (National Center for Biotechnology Information) and obtained from Integrated DNA Technologies. The cDNA was used as the template and the amplified product was detected by SYBR Green reagent (iQ SYBR Green Supermix, Bio-Rad). The primer specificity was tested using NCBI-Primer BLAST, melt curve analysis and in silico PCR (Bikandi et al., 2004). Data were normalized to endogenous control (16S rRNA) and comparative analyses of expression of candidate genes were determined using the comparative critical threshold (ΔΔCt) method on Quant Studio 3 real-time PCR system (Applied Biosystems, Thermo Fisher Scientific, Carlsbad, CA, USA).
Table 1.
Primers used for gene expression analysis using real-time quantitative PCR.
| Gene with accession no. | Primer | Sequence (5′–3′) |
|---|---|---|
| 16S-rRNA (NC_002163.1) | Forward | 5′-ATAAGCACCGGCTAACTCCG-3′ |
| (product length 78 bp) | Reverse | 5′-TTACGCCCAGTGATTCCGAG-3′ |
| luxS (NC_002163.1) | Forward | 5′-AGTGTTGCAAAAGCTTGGGA-3′ |
| (product length 106 bp) | Reverse | 5′-GCATTGCACAAGTTCCGCAT-3′ |
| motA (NC_002163.1) | Forward | 5′-AGCGGGTATTTCAGGTGCTT-3′ |
| (product length 75 bp) | Reverse | 5′-CCCCAAGGAGCAAAAAGTGC-3′ |
| motB (NC_002163.1) | Forward | 5′-AATGCCCAGAATGTCCAGCA-3′ |
| (product length 51 bp) | Reverse | 5′-AGTCTGCATAAGGCACAGCC-3′ |
| fliA (NC_002163.1) | Forward | 5′-AGCTTTCACGCCGTTACGAT-3′ |
| (product length 56 bp) | Reverse | 5′-TCTTGCAAAACCCCAGAAGT-3′ |
| ciaB (NC_002163.1) | Forward | 5′-TCTCAGCTCAAGTCGTTCCA-3′ |
| (product length 50 bp) | Reverse | 5′-GCCCGCCTTAGAACTTACAA-3′ |
| jlpA (NC_002163.1) | Forward | 5′-AGCACACAGGGAATCGACAG-3′ |
| (product length 66 bp) | Reverse | 5′-TAACGCTTCTGTGGCGTCTT-3′ |
| cadF (NC_002163.1) | Forward | 5′-CGCGGGTGTAAAATTCCGTC-3′ |
| (product length 135 bp) | Reverse | 5′-TCCTTTTTGCCACCAAAACCA-3′ |
Statistical Analyses
The Campylobacter mean CFUs were logarithmically transformed (Log CFU) to maintain the homogeneity of variance (Byrd et al., 2001). For all the in vitro experiments, duplicate samples were used and the assay was repeated three times. The data from trial 1 and trial 2 (in vivo study) and all in vitro experiments were pooled and analyzed using PROC MIXED procedure in SAS software (version 9.4, SAS Institute, Inc., Cary, NC, USA). The treatment means were separated by least square means, and a probability of P < 0.05 was required for statistical significance.
Results
Anti-Campylobacter Efficacy of BR in Chicken Cecal Contents In vitro
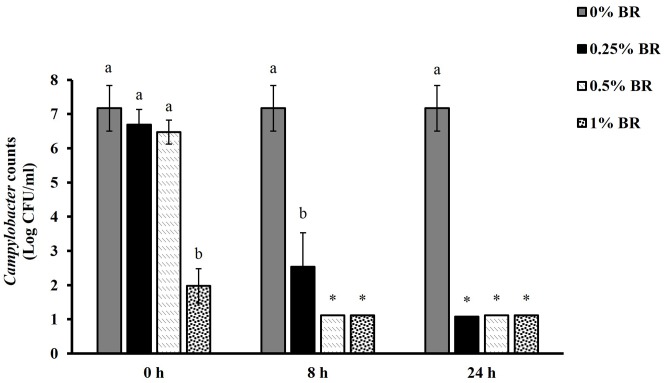
Figure 1 shows the effect of BR in reducing Campylobacter in chicken cecal contents in vitro. Among the various BR treatments, only 1% BR treatment significantly reduced Campylobacter counts at 0 h by 5.2 Log CFU/ml. At 8 h all the BR treatments significantly reduced Campylobacter populations in chicken cecal contents. Both 0.5 and 1% BR treatments reduced the counts below detection limit (1 Log CFU/ml) whereas 0.25% BR significantly reduced the counts by 4.6 Log CFU/ml compared to control. All the doses of BR reduced counts below detection limit after 24 h.
FIGURE 1.
Reduction of Campylobacter counts in cecal contents by different concentrations (0, 0.25, 0.50, and 1%) of BR at 0, 8, and 24 h. Results are averages of three independent experiments, each containing duplicate samples (mean and SEM). Bars with different letters represent a significant difference between treatments (P < 0.05). ∗Indicates Campylobacter counts below the detection limit (1 Log CFU/ml).
Effect of BR on C. jejuni Cecal Colonization and Average Body Weight Gain in Broiler Chickens
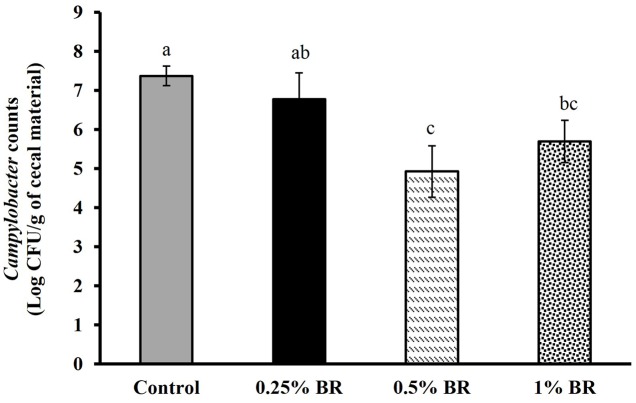
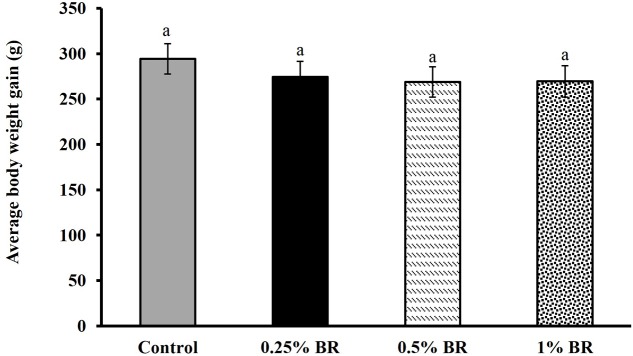
The effect of BR supplementation on Campylobacter cecal colonization in broilers is presented in Figure 2. In case of control, an average Campylobacter colonization of ∼7.5 Log CFU/g of cecal contents was observed on day 14. Supplementation of BR in feed at 0.5 and 1% level reduced cecal C. jejuni counts by ∼2.5 and 1.7 Log CFU/g, respectively as compared to the controls. However, BR supplementation at 0.25% did not significantly reduce C. jejuni counts in chickens. There was no significant difference in body weight gains in birds fed with BR compared to control birds at the end of 14 days (Figure 3).
FIGURE 2.
Effect of BR on cecal Campylobacter counts in 14 days old broiler chickens. Results are averages of two independent experiments, each containing 10 birds/treatments (mean and SEM). Bars with different letters represent a significant difference between treatments (P < 0.05).
FIGURE 3.
Effect of BR on body weight gain in broiler chickens. Results are averages of two independent experiments, each containing 10 birds/treatments (mean and SEM). Bars with different letters represent a significant difference between treatments (P < 0.05).
Estimation of Sub-inhibitory Concentration (SIC) of BR for Mechanistic Analysis
Based on growth curve analysis, the concentration of BR that did not significantly inhibit the growth of C. jejuni was 0.0125% and was selected as the SIC for subsequent mechanistic analysis (data not shown). Since DMSO was used as the diluent for BR, its effect on various colonization factors of C. jejuni was also studied.
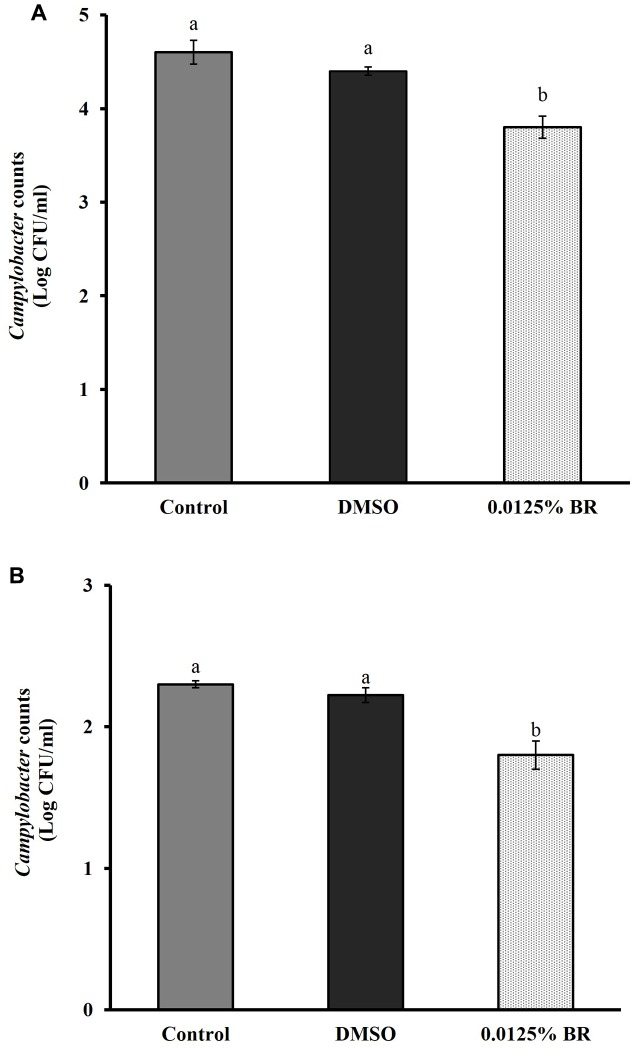
Effect of SIC of BR on C. jejuni Colonization Factors (Motility, Attachment, and Invasion of Caco-2 Cells)
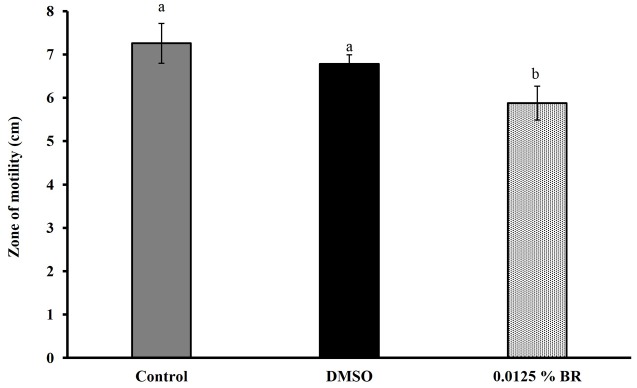
The addition of BR did not change the pH of motility medium or cell culture medium (P > 0.05). Figure 4 shows the effect of 0.0125% BR on C. jejuni motility. The SIC of BR reduced the zone of motility of C. jejuni to ∼5.9 cm (20% reduction) as compared to control that had a zone of ∼7.3 cm. In addition, BR also reduced C. jejuni attachment to and invasion of Caco-2 cells (Figures 5A,B). The adhesion of C. jejuni to Caco-2 cells was reduced by ∼0.7 Log CFU/ml (16%) as compared to control (Figure 5A). Similarly, C. jejuni invasion of Caco-2 cells was reduced by ∼0.5 Log CFU/ml (35%) as compared to control (Figure 5B). The DMSO treatment did not significantly affect the motility (Figure 4), adhesion (Figure 5A), or invasion of C. jejuni (Figure 5B). Taken together, these results show that BR exerts inhibitory effect on major colonization factors of C. jejuni.
FIGURE 4.
Effect of SIC of BR on the motility of Campylobacter jejuni. Results are averages of three independent experiments, each containing duplicate samples (mean and SEM). Bars with different letters represent a significant difference between treatments (P < 0.05).
FIGURE 5.
Effect of BR on C. jejuni (A) adhesion to and (B) invasion of human enterocytes. Results are averages of three independent experiments, each containing duplicate samples (mean and SEM). Bars with different letters represent a significant difference between treatments (P < 0.05).
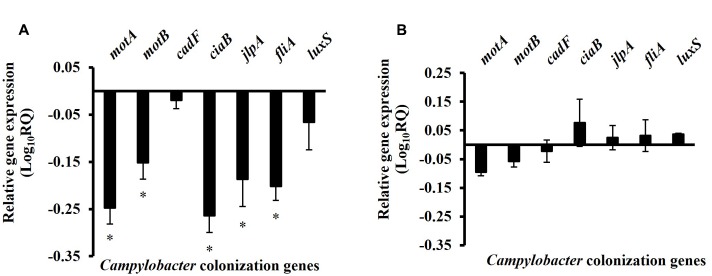
Effect of BR on Expression of C. jejuni Poultry Colonization Genes
The effect of SIC of BR on the expression of Campylobacter colonization genes is shown in Figure 6A. RT-qPCR revealed that BR reduced the transcription of genes critical for motility (motA, motB, fliA), adhesion and invasion (jlpA and ciaB) of Campylobacter as compared to control. However, other genes coding for quorum sensing (luxS) and attachment (cadF) were not significantly modulated. The DMSO treatment did not affect the expression of the tested genes (P > 0.05) (Figure 6B).
FIGURE 6.
The effect of (A) 0.0125% BR and (B) DMSO on the expression of chicken colonization genes of C. jejuni. 16S-rRNA served as endogenous control. Results are averages of three independent experiments, each containing duplicate samples (mean and SEM). ∗Indicates significantly down-regulated genes (P < 0.05).
Discussion
Despite substantial efforts, C. jejuni remains a leading biological contaminant of chicken products (Hermans et al., 2011b). Since Campylobacter resides primarily in the cecal crypts of birds, effective pre-harvest control strategy that reduces pathogen colonization in the cecal environment could potentially reduce the risk of fecal shedding and subsequent product contamination (Beery et al., 1988). An antimicrobial treatment that can be administered through feed represents a practical method for controlling pathogen colonization in birds. With increasing consumer demand for antibiotic free chickens, plant derived compounds or phytochemicals represent a large untapped resource that can serve as a safe and effective antibiotic alternative for controlling pathogens in birds.
Since degradation of chemicals in cecal contents is a potential concern, we investigated the anti-Campylobacter efficacy of BR in presence of cecal contents as a first step before conducting in vivo studies in broilers. We observed that BR is effective at reducing or eliminating C. jejuni in presence of cecal contents. The BR treatments reduced C. jejuni in a dose-dependent manner with highest tested dose (1%) showing significant reductions immediately (0 h), while the lower doses (0.25 and 0.5%) were effective after 8 h (Figure 1). Therefore, we selected these concentrations for testing their anti-Campylobacter efficacy in broilers.
Previous research has shown that a 2-Log reduction of Campylobacter counts from the poultry would produce up to 95% reduction in the risk of campylobacteriosis in humans (Nauta et al., 2016). In our study, C. jejuni colonized the birds effectively (∼7.5 Log CFU/g cecal contents in controls) and the compound consistently reduced C. jeuni colonization in both trials, therefore each data were combined (Figure 2). Supplementation of BR at 0.5 or 1.0% in feed significantly reduced enteric Campylobacter counts by at least 1.5 Log CFU/g when compared with positive control, indicating that BR could potentially reduce risk of subsequent human infections by reducing Campylobacter colonization in broilers. Previously, Upadhyaya et al. (2014) reported that supplementation of BR at 1% in feed reduced Salmonella enteritidis colonization in cecum, liver and crop by at least 1.5 Log CFU/g in 21 day broiler chickens suggesting that BR has a broad antimicrobial activity that includes major poultry associated foodborne pathogens. Structure-activity studies suggest that the antimicrobial activity of BR is associated with presence of carboxyl and hydroxyl groups on the phenol ring in its structure (Friedman et al., 2003).
Although found to be effective in controlling foodborne pathogen, selection of BR dosage is critical for optimal antimicrobial efficacy in poultry. We observed that feeding BR at level of 1.5% led to slower weight gain in birds (data not shown). Józefiak et al. (2010) reported that supplementation of benzoic acid (dehydroxylated form of BR) at 0.2% depressed the growth of broiler chickens. However, in another study, supplementation of benzoic acid at 0.1% in feed improved the performance in turkey poults (Giannenas et al., 2014). These researchers also observed an increase in lactic acid bacteria and decrease in coliform bacteria in the ceca. There are only a few studies on the absorption, metabolism, and effect of BR on poultry gut. Beta-resorcylic acid has a moderate dissociation constant similar to benzoic acid, and as a weak acid remains in a non-dissociated form in the stomach and intestine (Mattoo, 1959; Milne, 1965). Therefore, it might be acting through similar mechanism(s). It is possible that feeding higher concentration of BR could modulate poultry gut environment leading to reduced appetite and suppressed growth in chickens.
To investigate the potential mechanism of action of BR on Campylobacter, we evaluated the effect of BR at its SIC, on various virulence attributes of C. jejuni. C. jejuni strain S-8 was randomly selected from the four-strains for mechanistic analysis. Since SICs are not bacteriostatic or bactericidal, the results we observed in our phenotypic assays are not due to killing of Campylobacter but potentially due to modulation of its pathophysiology. Motility of Campylobacter is one of the essential factors for colonization in poultry gut (Morooka et al., 1985; Hermans et al., 2011a). We found that presence of BR in the medium reduced Campylobacter motility as compared to controls. Similar results of reduced Campylobacter motility were observed when Campylobacter was exposed to citrus extract (Castillo et al., 2014) and berries (Salaheen et al., 2014). Since a chicken cecal epithelial cell line is not commercially available, we used the well-established human epithelial cells (Caco-2) for conducting attachment and invasion assays. Previously, Byrne et al. (2007) showed that C. jejuni attaches and invades both human epithelial cells and primary chicken enterocytes with similar efficiency. Our results from the cell culture revealed that BR reduced C. jejuni adhesion and invasion of human enterocytes by 0.7 and 0.5 Log CFU/ml respectively compared to control. This reduction was similar to that observed with other phytochemicals such as berry extracts (Salaheen et al., 2014), and extracts from Acacia farnesiana, Artemisia ludoviciana, Opuntia ficus-indica, and Cynara scolymus (Castillo et al., 2014). Since Campylobacter adhesion to epithelial cells is an important step for colonization (Jin et al., 2001; Hermans et al., 2011a), a reduction in this virulence attribute could potentially reduce colonization in birds. Bezek et al. (2016) had similar findings with extracts from Euodia ruticarpa. In addition, these researchers observed that other virulence attributes, including biofilm formation and quorum sensing were also affected by the phytochemical.
It is previously reported that the SIC of antimicrobials modulates the expression of various virulence proteins and associated genes in bacteria thereby resulting in changes in their pathophysiology and virulence (Goh et al., 2002; Tsui et al., 2004; Qiu et al., 2010; Upadhyay et al., 2012, 2014; Maisuria et al., 2016). To study if similar mechanisms exist in Campylobacter, we investigated the effect of BR on various genes of Campylobacter that are known to facilitate colonization in poultry. motA, motB, and fliA genes are critical for Campylobacter motility (Nachamkin et al., 1993; Wassenaar et al., 1993; Fernando et al., 2007). motA and motB code for flagella motor protein, while fliA codes for flagella biosynthesis protein. Similarly, cadF along with the genes jlpA and ciaB facilitate C. jejuni adherence onto the intestinal cells (Hermans et al., 2011a). The transcription level of genes coding for motility (motA, motB, fliA) and attachment (jlpA, ciaB) was downregulated as revealed in RT-qPCR results, thus indicating that the anti-Campylobacter colonization effect observed with BR could be mediated via downregulation of critical colonization genes in the pathogen.
Conclusion
β-resorcylic acid supplementation in feed reduced Campylobacter colonization in birds without affecting their body weight gain or feed conversion ratio. Mechanistic analysis using standard bioassays, cell culture and gene expression analysis showed that the reduction in Campylobacter colonization in birds could at least partially be attributed to modulation of critical colonization factors, virulence proteins and associated genes in the pathogen. Thus, BR could potentially be used as a feed supplement to control C. jejuni colonization in broiler chickens. Although, the results from this study are encouraging, follow-up studies investigating the efficacy of BR in market age birds and cost-benefit analysis of feed application are warranted.
Disclaimer
Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USDA and does not imply its approval to the exclusion of other products that may be suitable.
Author Contributions
BW and DD designed the study. BW, AU, KA, SS conducted the experiments. BW and AU wrote the manuscript. KV, AD, and DD critically analyzed and revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This research was funded in part by the USDA-NIFA-OREI 2011-01955.
References
- Achen M., Morishita T. Y., Ley E. C. (1998). Shedding and colonization of Campylobacter jejuni in broilers from day-of-hatch to slaughter age. Avian Dis. 42 732–737. 10.2307/1592708 [DOI] [PubMed] [Google Scholar]
- Annan-Prah A., Janc M. (1988). The mode of spread of Campylobacter jejuni/coli to broiler flocks. J. Vet. Med. 35 11–18. 10.1111/j.1439-0450.1988.tb00461.x [DOI] [PubMed] [Google Scholar]
- Arsenault J., Letellier A., Quessy S., Boulianne M. (2007). Prevalence and risk factors for Salmonella and Campylobacter spp. carcass contamination in broiler chickens slaughtered in Quebec. Can. J. Food Prot. 70 1820–1828. 10.4315/0362-028X-70.8.1820 [DOI] [PubMed] [Google Scholar]
- Arsi K., Donoghue A. M., Woo-Ming A., Blore P. J., Donoghue D. J. (2015). The efficacy of selected probiotic and prebiotic combinations in reducing Campylobacter colonization in broiler chickens. J. Appl. Poult. Res. 24 327–334. 10.3382/japr/pfv032 [DOI] [Google Scholar]
- Baskaran S. A., Upadhyay A., Kollanoor Johny A., Upadhyaya I., Mooyottu S., Amalaradjou M. A. R., et al. (2013). Efficacy of plant-derived antimicrobials as antimicrobial wash treatments for reducing enterohemorrhagic Escherichia Coli O157: H7 on apples. J. Food Sci. 78 M1399–M1404. 10.1111/1750-3841.12174 [DOI] [PubMed] [Google Scholar]
- Beery J. T., Hugdahl M. B., Doyle M. P. (1988). Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 54 2365–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrang M. E., Buhr R. J., Cason J. A. (2000). Campylobacter recovery from external and internal organs of commercial broiler carcass prior to scalding. Poult. Sci. 79 286–290. 10.1093/ps/79.2.286 [DOI] [PubMed] [Google Scholar]
- Bezek K., Kurinèiè M., Knauder E., Klanènik A., Raspor P., Bucar F., et al. (2016). Attenuation of adhesion, biofilm formation and quorum sensing of Campylobacter jejuni by Euodia ruticarpa. Phytother. Res. 30 1527–1532. 10.1002/ptr.5658 [DOI] [PubMed] [Google Scholar]
- Bikandi J., San Millán R., Rementeria A., Garaizar J. (2004). In silico analysis of complete bacterial genomes: PCR, AFLP-PCR, and endonuclease restriction. Bioinformatics 20 798–799. 10.1093/bioinformatics/btg491 [DOI] [PubMed] [Google Scholar]
- Black R. E., Levine M. M., Clements M. L., Hughes T. P., Blaser M. J. (1988). Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157 472–479. 10.1093/infdis/157.3.472 [DOI] [PubMed] [Google Scholar]
- Boysen L., Nauta M., Rosenquist H. (2016). Campylobacter spp. and Escherichia coli contamination of broiler carcasses across the slaughter line in Danish slaughterhouses. Microb. Risk Anal. 2 63–67. 10.1016/j.mran.2016.05.005 [DOI] [Google Scholar]
- Buckley A. M., Wang J., Hudson D. L., Grant A. J., Jones M. A., Maskell D. J., et al. (2010). Evaluation of live-attenuated Salmonella vaccines expressing Campylobacter antigens for control of C. jejuni in poultry. Vaccine 28 1094–1105. 10.1016/j.vaccine.2009.10.018 [DOI] [PubMed] [Google Scholar]
- Burt S. (2004). Essential oils: their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol. 94 223–253. 10.1016/j.ijfoodmicro.2004.03.022 [DOI] [PubMed] [Google Scholar]
- Byrd J. A., Hargis B. M., Caldwell D. J., Bailey R. H., Herron K. L., McReynolds J. L., et al. (2001). Effect of lactic acid administration in the drinking water during preslaughter feed withdrawal on Salmonella and Campylobacter contamination of broilers. Poult. Sci. 80 278–283. 10.1093/ps/80.3.278 [DOI] [PubMed] [Google Scholar]
- Byrne C. M., Clyne M., Bourke B. (2007). Campylobacter jejuni adhere to and invade chicken intestinal epithelial cells in vitro. Microbiology 153 561–569. 10.1099/mic.0.2006/000711-0 [DOI] [PubMed] [Google Scholar]
- Carrillo C. L., Atterbury R. J., El-Shibiny A., Connerton P. L., Dillon E., Scott A., et al. (2005). Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 71 6554–6563. 10.1128/AEM.71.11.6554-6563.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo S., Heredia N., Arechiga-Carvajal E., García S. (2014). Citrus extracts as inhibitors of quorum sensing, biofilm formation and motility of Campylobacter jejuni. Food Biotechnol. 28 106–122. 10.1080/08905436.2014.895947 [DOI] [Google Scholar]
- Chintoan-Uta C., Cassady-Cain R. L., Stevens M. P. (2016). Evaluation of flagellum-related proteins FliD and FspA as subunit vaccines against Campylobacter jejuni colonisation in chickens. Vaccine 34 1739–1743. 10.1016/j.vaccine.2016.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K., Farnell M. B., Donoghue A. M., Stern N. J., Svetoch E. A., Eruslanov B. N., et al. (2006). Bacteriocins reduce Campylobacter colonization and alter gut morphology in turkey poults. Poult. Sci. 85 1570–1575. 10.1093/ps/85.9.1570 [DOI] [PubMed] [Google Scholar]
- Crim S. M., Griffin P. M., Tauxe R., Marder E. P., Gilliss D., Cronquist A. B., et al. (2015). Preliminary incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 US sites, 2006–2014. MMWR Morb. Mortal. Wkly. Rep. 64 495–499. [PMC free article] [PubMed] [Google Scholar]
- Danis K., Di Renzi M., O’Neill W., Smyth B., McKeown P., Foley B., et al. (2009). Risk factors for sporadic Campylobacter infection: an all-Ireland case-control study. Euro Surveill. 14 19123. [PubMed] [Google Scholar]
- Dhillon A. S., Shivaprasad H. L., Schaberg D., Wier F., Weber S., Bandli D. (2006). Campylobacter jejuni infection in broiler chickens. Avian Dis. 50 55–58. 10.1637/7411-071405R.1 [DOI] [PubMed] [Google Scholar]
- Fernando U., Biswas D., Allan B., Willson P., Potter A. A. (2007). Influence of Campylobacter jejuni fliA, rpoN and flgK genes on colonization of the chicken gut. Int. J. Food Microbiol. 118 194–200. 10.1016/j.ijfoodmicro.2007.07.038 [DOI] [PubMed] [Google Scholar]
- Friedman C. R., Hoekstra R. M., Samuel M., Marcus R., Bender J., Shiferaw B., et al. (2004). Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin. Infect. Dis. 38 S285–S296. 10.1086/381598 [DOI] [PubMed] [Google Scholar]
- Friedman M., Henika P. R., Mandrell R. E. (2003). Antibacterial activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 66 1811–1821. 10.4315/0362-028X-66.10.1811 [DOI] [PubMed] [Google Scholar]
- Giannenas I., Papaneophytou C. P., Tsalie E., Pappas I., Triantafillou E., Tontis D., et al. (2014). Dietary supplementation of benzoic acid and essential oil compounds affects buffering capacity of the feeds, performance of turkey poults and their antioxidant status, pH in the digestive tract, intestinal microbiota and morphology. Asian Australas. J. Anim. Sci. 27 225–236. 10.5713/ajas.2013.13376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh E. B., Yim G., Tsui W., McClure J., Surette M. G., Davies J. (2002). Transcriptional modulation of bacterial gene expression by sub inhibitory concentrations of antibiotics. Proc. Natl. Acad. Sci. U.S.A. 99 17025–17030. 10.1073/pnas.252607699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradel K. O., Nielsen H. L., Schønheyder H. C., Ejlertsen T., Kristensen B., Nielsen H. (2009). Increased short- and long-term risk of inflammatory bowel disease after Salmonella or Campylobacter gastroenteritis. Gastroenterology 137 495–501. 10.1053/j.gastro.2009.04.001 [DOI] [PubMed] [Google Scholar]
- Herman L., Heyndrickx M., Grijspeerdt K., Vandekerchove D., Rollier I., De Zutter L. (2003). Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol. Infect. 131 1169–1180. 10.1017/S0950268803001183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D., Van Deun K., Martel A., Van Immerseel F., Messens W., Heyndrickx M., et al. (2011a). Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 42:82 10.1186/1297-9716-42-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D., Van Deun K., Messens W., Martel A., Van Immerseel F., Haesebrouck F., et al. (2011b). Campylobacter control in poultry by current intervention measures ineffective: urgent need for intensified fundamental research. Vet. Microbiol. 152 219–228. 10.1016/j.vetmic.2011.03.010 [DOI] [PubMed] [Google Scholar]
- Holley R. A., Patel D. (2005). Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 22 273–292. 10.1016/j.fm.2004.08.006 [DOI] [Google Scholar]
- Humphery T. J., Henley A., Lanning D. G. (1993). The colonization of broiler chickens with Campylobacter jejuni: some epidemiological investigations. Epidemiol. Infect. 110 601–607. 10.1017/S0950268800051025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Joe A., Lynett J., Hani E. K., Sherman P., Chan V. L. (2001). JlpA, a novel surface-exposed lipoprotein specific to Campylobacter jejuni, mediates adherence to host epithelial cells. Mol. Microbiol. 39 1225–1236. 10.1111/j.1365-2958.2001.02294.x [DOI] [PubMed] [Google Scholar]
- Józefiak D., Kaczmarek S., Rutkowski A. (2010). The effects of benzoic acid supplementation on the performance of broiler chickens. J. Anim. Physiol. Anim. Nutr. 94 29–34. 10.1111/j.1439-0396.2008.00875.x [DOI] [PubMed] [Google Scholar]
- Kollanoor Johny A., Darre M. J., Donoghue A. M., Donoghue D. J., Venkitanarayanan K. (2010). Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella Enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J. Appl. Poult. Res. 19 237–244. 10.3382/japr.2010-00181 [DOI] [Google Scholar]
- Line J. E. (2001). Development of a selective differential agar for isolation and enumeration of Campylobacter spp. J. Food Prot. 64 1711–1715. 10.4315/0362-028X-64.11.1711 [DOI] [PubMed] [Google Scholar]
- Maisuria V. B., Lopez-de Los Santos Y., Tufenkji N., Déziel E. (2016). Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci. Rep. 6:30169 10.1038/srep30169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangen M. J., Havelaar A. H., Haagsma J. A., Kretzschmar M. E. E. (2016). The burden of Campylobacter-associated disease in six European countries. Microb. Risk Anal. 2-3 48–52. 10.1016/j.mran.2016.04.001 [DOI] [Google Scholar]
- Mattoo B. (1959). On the complete dissociation of 2, 4-dihydroxybenzoic (β-resorcyclic) acid. Z. Phys. Chem. 22 187–198. 10.1524/zpch.1959.22.3_4.187 [DOI] [Google Scholar]
- Mattson T. E., Kollanoor Johny A., Amalaradjou M. A. R., More K., Schreiber D. T., Patel J., et al. (2011). Inactivation of Salmonella spp. on tomatoes by plant molecules. Int. J. Food Microbiol. 144 464–468. 10.1016/j.ijfoodmicro.2010.10.035 [DOI] [PubMed] [Google Scholar]
- Mead P. S., Slutsker L., Dietz V., McCaig L. F., Bresee J. S., Shapiro C., et al. (1999). Food-related illness and death in the United States. Emerg. Infect. Dis. 5 607–625. 10.3201/eid0505.990502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne M. (1965). Influence of acid-base balance on efficacy and toxicity of drugs. Proc. R. Soc. Med. 58 961. [PMC free article] [PubMed] [Google Scholar]
- Moroni O., Kheadr E., Boutin Y., Lacroix C., Fliss I. (2006). Inactivation of adhesion and invasion of food-borne Listeria monocytogenes by bacteriocin-producing Bifidobacterium strains of human origin. Appl. Environ. Microbiol. 72 6894–6901. 10.1128/AEM.00928-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morooka T., Umeda A., Amako K. (1985). Motility as an intestinal colonization factor for Campylobacter jejuni. Microbiology 131 1973–1980. 10.1099/00221287-131-8-1973 [DOI] [PubMed] [Google Scholar]
- Nachamkin I., Yang X. H., Stern N. J. (1993). Role of Campylobacter jejuni flagella as colonization factors for three-day-old chicks: analysis with flagellar mutants. Appl. Environ. Microbiol. 59 1269–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta M., Johannessen G., Adame L. L., Williams N., Rosenquist H. (2016). The effect of reducing numbers of Campylobacter in broiler intestines on human health risk. Microb. Risk Anal. 2-3 68–77. 10.1016/j.mran.2016.02.001 [DOI] [Google Scholar]
- Qiu J., Feng H., Lu J., Xiang H., Wang D., Dong J., et al. (2010). Eugenol reduces the expression of virulence-related exoproteins in Staphylococcus aureus. Appl. Environ. Microbiol. 76 5846–5851. 10.1128/AEM.00704-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich F., Atanassova V., Haunhorst E., Klein G. (2008). The effects of Campylobacter numbers in caeca on the contamination of broiler carcasses with Campylobacter. Int. J. Food Microbiol. 127 116–120. 10.1016/j.ijfoodmicro.2008.06.018 [DOI] [PubMed] [Google Scholar]
- Rosenquist H., Nielsen N. L., Sommer H. M., Nørrung B., Christensen B. B. (2003). Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int. J. Food Microbiol. 83 87–103. 10.1016/S0168-1605(02)00317-3 [DOI] [PubMed] [Google Scholar]
- Salaheen S., Nguyen C., Hewes D., Biswas D. (2014). Cheap extraction of antibacterial compounds of berry pomace and their mode of action against the pathogen Campylobacter jejuni. Food Control 46 174–181. 10.1016/j.foodcont.2014.05.026 [DOI] [Google Scholar]
- Samuel M. C., Vugia D. J., Shallow S., Marcus R., Segler S., McGivern T., et al. (2004). Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996–1999. Clin. Infect. Dis. 38 S165–S174. 10.1086/381583 [DOI] [PubMed] [Google Scholar]
- Santini C., Baffoni L., Gaggia F., Granata M., Gasbarri R., Di Gioia D., et al. (2010). Characterization of probiotic strains: an application as feed additives in poultry against Campylobacter jejuni. Int. J. Food Microbiol. 141 S98–S108. 10.1016/j.ijfoodmicro.2010.03.039 [DOI] [PubMed] [Google Scholar]
- Shrestha S., Arsi K., Wagle B. R., Donoghue A. M., Donoghue D. J. (2017). The ability of select probiotics to reduce enteric Campylobacter colonization in broiler chickens. Int. J. Poult. Sci. 16 37–42. 10.3923/ijps.2017.37.42 [DOI] [Google Scholar]
- Spiller R. C. (2007). Role of infection in irritable bowel syndrome. J. Gastroenterol. 42(Suppl. 17), 41–47. 10.1007/s00535-006-1925-8 [DOI] [PubMed] [Google Scholar]
- Stern N. J., Bailey J. S., Blankenship L. C., Cox N. A., McHan F. (1988). Colonization characteristics of Campylobacter jejuni in chick ceca. Avian Dis. 32 330–334. 10.2307/1590822 [DOI] [PubMed] [Google Scholar]
- Stern N. J., Svetoch E. A., Eruslanov B. V., Kovalev Y. N., Volodina L. I., Perelygin V. V.et al. (2005). Paenibacillus polymyxa purified bacteriocin to control Campylobacter jejuni in chickens. J. Food Prot. 68 1450–1453. 10.4315/0362-028X-68.7.1450 [DOI] [PubMed] [Google Scholar]
- Svetoch E. A., Stern N. J. (2010). Bacteriocins to control Campylobacter spp. in poultry-A review. Poult. Sci. 89 1763–1768. 10.3382/ps.2010-00659 [DOI] [PubMed] [Google Scholar]
- Tsui W. H., Yim G., Wang H. H., McClure J. E., Surette M. G., Davies J. (2004). Dual effects of MLS antibiotics: transcriptional modulation and interactions on the ribosome. Chem. Biol. 11 1307–1316. 10.1016/j.chembiol.2004.07.010 [DOI] [PubMed] [Google Scholar]
- U. S. Food and Drug Administration (2013). Everything Added to Food in the United States. Available at: http://www.accessdata.fda.gov/scripts/fcn/fcnDetailNavigation.cfm?rpt=eafuslisting\&id=2473 [accessed July 01 2017]. [Google Scholar]
- Upadhyay A., Kollanoor Johny A., Amalaradjou M. A. R., Baskaran S. A., Kim K. S., Venkitanarayanan K. (2012). Plant-derived antimicrobials reduce Listeria monocytogenes virulence factors in vitro, and down-regulate expression of virulence genes. Int. J. Food Microbiol. 157 88–94. 10.1016/j.ijfoodmicro.2012.04.018 [DOI] [PubMed] [Google Scholar]
- Upadhyay A., Upadhyaya I., Kollanoor Johny A., Baskaran S. A., Mooyottu S., Karumathil D., et al. (2013). Inactivation of Listeria monocytogenes on frankfurters by plant derived antimicrobials alone or in combination with hydrogen peroxide. Int. J. Food Microbiol. 163 114–118. 10.1016/j.ijfoodmicro.2013.01.023 [DOI] [PubMed] [Google Scholar]
- Upadhyay A., Upadhyaya I., Kollanoor-Johny A., Venkitanarayanan K. (2014). Combating pathogenic microorganisms using plant-derived antimicrobials: a minireview of the mechanistic basis. Biomed. Res. Int. 2014:761741 10.1155/2014/761741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya I., Upadhyay A., Yin H., Nair M., Karumathil D. P., Bhattaram V. K., et al. (2014). Effect of β –resorcylic acid and chitosan on reducing Salmonella Enteritidis colonization in 21-day-old broiler chicks. Poult. Sci. 93(E-Suppl. 1), 70. [Google Scholar]
- Wagenaar J. A., Van Bergen M. A., Mueller M. A., Wassenaar T. M., Carlton R. M. (2005). Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet. Microbiol. 109 275–283. 10.1016/j.vetmic.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Wassenaar T. M., van der Zeijst B. A., Ayling R., Newell D. G. (1993). Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. Microbiology 139 1171–1175. 10.1099/00221287-139-6-1171 [DOI] [PubMed] [Google Scholar]
- Woodall C. A., Jones M. A., Barrow P. A., Hinds J., Marsden G. L., Kelly D. J., et al. (2005). Campylobacter jejuni gene expression in the chick cecum: evidence for adaptation to a low-oxygen environment. Infect. Immun. 73 5278–5285. 10.1128/IAI.73.8.5278-5285.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]