Figure 1.
Characterization of γ1D316A Mutation In Vitro
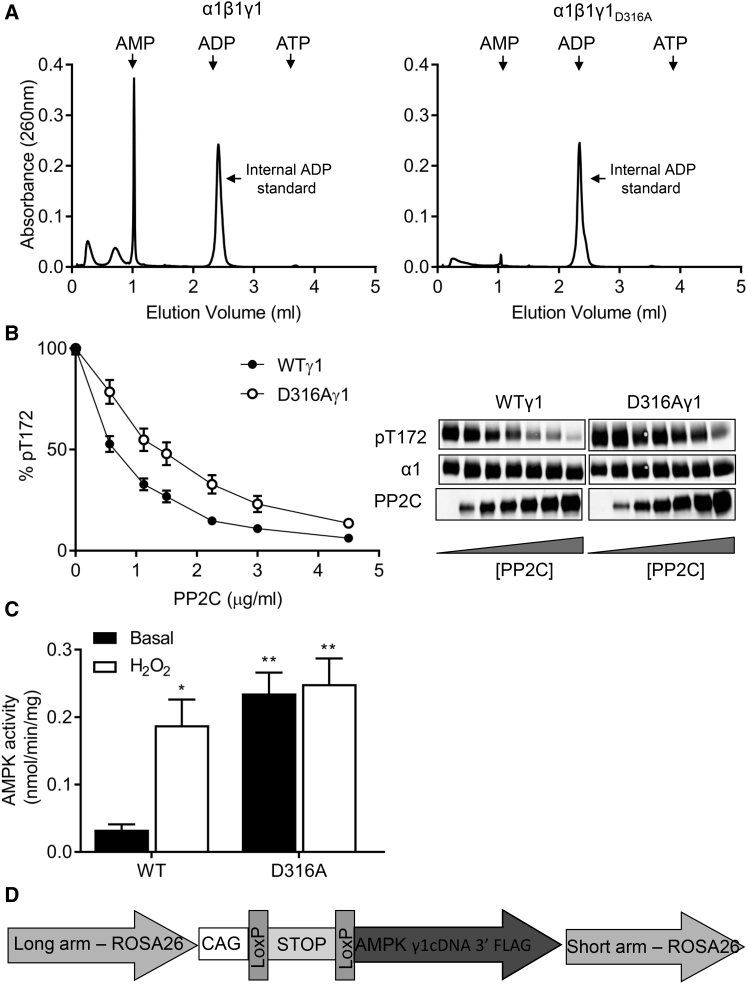
(A) AMP in perchloric acid extracts of bacterially expressed AMPK complexes (wild-type α1β1γ1 or α1β1γ1D316A) was determined by ion-exchange chromatography. The elution positions of AMP, ADP, and ATP standards are indicated by the arrows. ADP (4 nmol) was added prior to extraction as an internal standard and is marked by an arrow.
(B) Following phosphorylation by CaMKKβ, recombinant AMPK complexes were incubated in the presence of increasing concentrations of Protein phosphatase 2C (PP2C) for 20 min and then analyzed for T172 phosphorylation by western blotting. A representative blot showing the level of T172 phosphorylation, total AMPKα1, and PP2C is shown. Quantification of T172 phosphorylation relative to a control (in the absence of PP2C) is shown (n = 4).
(C) COS7 cells were co-transfected with cDNAs encoding α1, β1, and either wild-type or D316A γ1, harboring a C-terminal Flag epitope tag. AMPK activity from cells treated with or without 1 mM H2O2 for 15 min was measured in immune-complexes isolated with anti-Flag antibody (n = 4, ∗p < 0.05, ∗∗p < 0.01).
(D) Schematic representation of the construct used to generate transgenic mice expressing either WTγ1 or D316Aγ1.