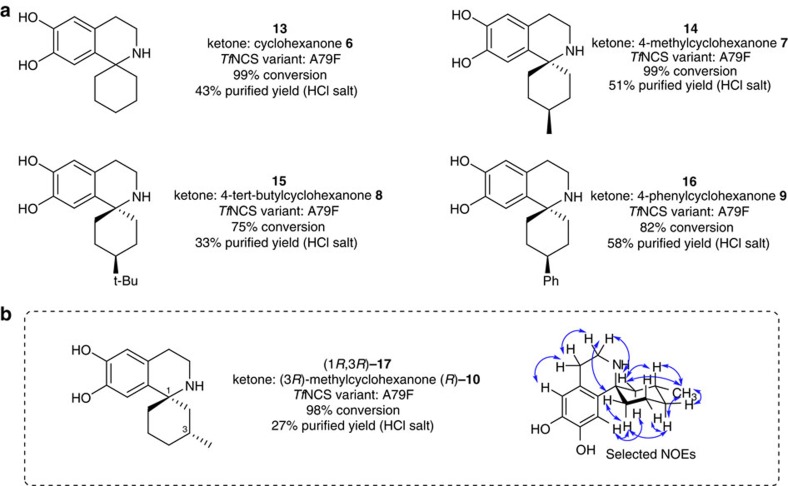
Figure 6. Biocatalytic formation of spiro-1,1′-disubstituted THIQs using Δ29TfNCS variants.
(a) Spiro-THIQ products isolated from biotransformations. All compounds feature a fixed stereocentre at the C-4 position. (b) Chiral (R)-Spiro-THIQ product (1R,3R)-17 isolated from biotransformation. Important NOE (nuclear Overhauser effect) correlations are highlighted. See Supplementary Methods for full synthetic procedures, Supplementary Figs 9 and 10 for HPLC analysis and Supplementary Figs 14-18 for NMR spectra.