The current redetermination confirms the previous structure report, but with considerably higher precision and accuracy.
Keywords: crystal structure, redetermination, organotin compound, acetylacetonate
Abstract
The redetermination of the title compound, [Sn(CH3)2(C5H7O2)2] or SnMe2(acac)2, from CCD data recorded at 100 K basically confirms the previous study based on integrated film data recorded at room temperature [Miller & Schlemper (1972 ▸). Inorg. Chem. 12, 677–681], but reveals a remarkable shrinkage of the a axis [7.12 (1) > 6.7694 (4) Å]. The molecule belongs to point group Ci with the SnIV atom on a centre of inversion. The SnIV atom shows a slightly distorted octahedral coordination sphere with the methyl groups in trans positions and a Sn—C bond length of 2.115 (2) Å which may serve as a standard value for an Sn—CH3 bond of an octahedrally coordinated SnIV atom. The Sn—O bonds involving the two carbonyl groups of the acetylacetonate ligand are of equal length [2.180 (1) and 2.183 (1) Å], as are the C=O [1.273 (1) and 1.274 (1) Å] and C—C bond lengths [1.393 (2) and 1.400 (2) Å]. The acetylacetonate ligand deviates considerably from planarity, with a dihedral angle of 5.57 (9)° between the least-squares planes of the two acetone moieties. The four O atoms of the two symmetry-related acetylacetonate ligands are arranged in a nearly quadratic rectangle. Weak C—H⋯O interactions consolidate the crystal packing.
Chemical context
The crystal structure of the title compound, [Sn(CH3)2(C5H7O2)2] or SnMe2(acac)2, was determined in the early 1970s at room temperature by visual estimation of film data and refined to a final conventional R value of 0.079 (Miller & Schlemper, 1972 ▸). All bond lengths and angles of the original study seem chemically reasonable but accuracy suffers from the limited precision of that kind of data collection. As SnMe2(acac)2 serves as a reference for all diorganotin(IV) diacetylacetonates and bis-1,3-diketonates in general, we decided to redetermine its structure from CCD data recorded at low temperature. Moreover, the title compound is an excellent candidate for the determination of the Sn—CMe bond length as another reference in case the SnIV atom is in a well-defined octahedral coordination. The precise measurement of this Sn—C distance therefore should supplement the observations of Britton (2006 ▸) who found a significant change in Sn—C bond lengths depending on the organic moiety attached to an SnIV atom.
Structural commentary
The redetermination of the crystal structure of the title compound confirms the former results obtained by Miller & Schlemper (1973 ▸) with respect to the chosen space group (P21/n) and the constitution of the asymmetric unit comprising half a formula unit with the Sn atom at a crystallographic centre of inversion [Wyckhoff symbol: b]. As we performed the X-ray measurement at 100 K, the unit-cell volume is somewhat smaller in comparison with the original room-temperature data which is mainly caused by a considerable change of the a axis from 7.12 (1) to 6.7694 (4) Å while changes of all other lattice parameters [b
original = 13.87 (2), c
original = 7.69 (1) Å, β
original = 104.7 (2)°] show a normal temperature-dependent shrinkage.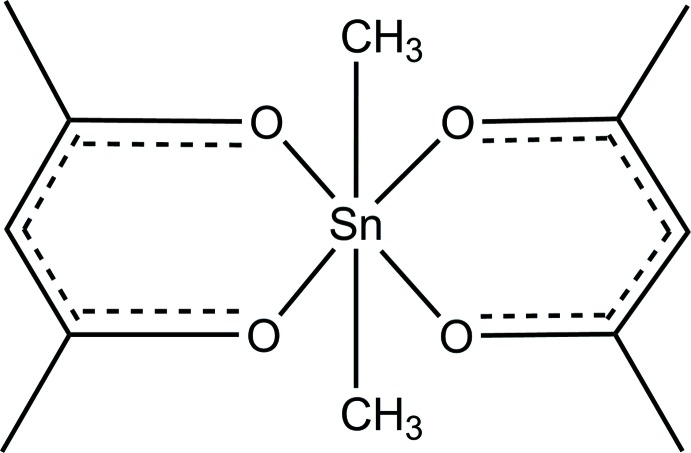
The Sn atom is octahedrally coordinated with the two methyl groups in a trans position (Fig. 1 ▸, Table 1 ▸). The Sn—C bond shows a length of 2.115 (2) Å and is significantly shorter than the value [2.14 (2) Å] obtained by Miller & Schlemper (1972 ▸). Because of the higher coordination number, the value differs to some extent from the value of 2.099 (2) Å for Sn with a coordination number of four as observed in dimethyldithiocyanato tin(IV) (Britton, 2006 ▸). The two bonds between the Sn atom and the two different O atoms of the acetylacetonate ligand are of equal length [2.180 (1)/2.183 (1) Å]. In accordance with the almost symmetrical bonding of the acetylacetonate ligand to tin, C—O [1.273 (2)/1.274 (2) Å] and C—C [1.393 (2)/1.400 (2) Å] bonds of the 1,3-diketonate skeleton are of equal length. Although these values are typical for a delocalized π system, the atoms in question show a significant deviation from planarity at the central C2 atom, resulting in a dihedral angle of 5.57 (9)° between the least-squares planes defined by O1/C1/C2/C3 [deviations from planarity: 0.003 (1), −0.007 (1), 0.002 (1), 0.002 (1) Å, respectively] and O2/C3/C2/C4 [deviations from planarity: 0.002 (1), −0.004 (1), 0.002 (1), 0.001 (2) Å, respectively] (Fig. 2 ▸). Moreover, all bond angles in the six-membered chelate ring are considerably larger [125.61 (9)° at O1, 126.2 (1)° at C1, 128.4 (1)° at C2, 126.07 (13)° at C3, 125.68 (9)° at O2] than expected for sp 2-hybridized atoms, with exception of the bond angle at Sn1 that amounts to 85.99 (4)°. The four O atoms of the two symmetry-related acetylacetonate ligands around the Sn atom form a planar rectangle with similar edge lengths [O1⋯O2 = 2.975 (1)/O1⋯O1 = 3.191 (1) Å], and almost right angles [89.9 (1)° at O1 and 90.1 (1)° at O2]. This plane is nearly perpendicular to the axis through the two methyl groups [deviation: 0.44 (2)°] but constitutes a dihedral angle of 10.2 (1)° with the least-squares plane through the two carbonyl groups of the acetylacetonate ligand [deviations from planarity: O1 = 0.006 (1), C1 = −0.007 (1), O2 = −0.006 (1) Å, C3 = 0.007 (1) Å] (Fig. 3 ▸).
Figure 1.
The molecular structure of the title compound, showing the atom-labeling scheme of the asymmetric unit. Displacement ellipsoids are drawn at the 50% probability level.
Table 1. Selected geometric parameters (Å, °).
| Sn1—C6 | 2.115 (2) | O1—C1 | 1.274 (2) |
| Sn1—C6i | 2.115 (2) | O2—C3 | 1.273 (2) |
| Sn1—O1 | 2.180 (1) | C1—C2 | 1.393 (2) |
| Sn1—O1i | 2.180 (1) | C2—C3 | 1.400 (2) |
| Sn1—O2 | 2.183 (1) | C3—C5 | 1.499 (2) |
| Sn1—O2i | 2.183 (1) | C1—C4 | 1.505 (2) |
| C6i—Sn1—C6 | 180.0 | C1—O1—Sn1 | 125.61 (9) |
| C6—Sn1—O1 | 90.20 (5) | O1—C1—C2 | 126.2 (1) |
| C6—Sn1—O2 | 90.41 (5) | C1—C2—C3 | 128.4 (1) |
| O1—Sn1—O2 | 85.99 (4) | C3—O2—Sn1 | 125.68 (9) |
Symmetry code: (i)  .
.
Figure 2.
Twisting of the acetylacetonate ligand at atom C2 with respect to the least-squares planes (green dashed lines) O1/C1/C2/C5 and O2/C3/C2/C4 in a view parallel to these planes. Non-H atoms are shown as displacement ellipsoids at the 50% probability level.
Figure 3.
Orientation of the acetylacetate ligand (least-squares plane through both carbonyl groups) in relation to the plane defined by the four O atoms coordinating to the SnIV atom. The view is parallel to these planes (green lines). Non-H atoms are shown as displacement ellipsoids at the 50% probability level.
Supramolecular features
In the absence of classical H-donor groups, intermolecular interactions are restricted to van der Waals and weak O⋯H—C interactions. The most prominent ones are associated with the methyl hydrogen atoms H42 and H51 of the acetylacetonate ligand as they interact simultaneously with both oxygen atoms of neighbouring molecules [C42⋯O2i = 2.906 Å, C42⋯O1ii = 2.852 Å, C51⋯O2iii = 2.797 Å, H51⋯O1iv = 2.850 Å; symmetry codes: (i) x, y, 1 + z; (ii) 1 − x, 1 − y,1 − z; (iii)  + x,
+ x,  − y,
− y,  + z; (iv)
+ z; (iv)  − x, −
− x, − + y,
+ y,  + z] (Fig. 4 ▸). These interactions are completed by a third O⋯H—C contact of similar length [H2⋯O2iii = 2.893 Å, H43⋯O1v = 2.992 Å, symmetry code: (v) 2 − x, 1 − y, 1 − z] for each oxygen atom. In summary, the intermolecular contacts result in a columnar arrangement of the molecules parallel to the a axis (Fig. 5 ▸).
+ z] (Fig. 4 ▸). These interactions are completed by a third O⋯H—C contact of similar length [H2⋯O2iii = 2.893 Å, H43⋯O1v = 2.992 Å, symmetry code: (v) 2 − x, 1 − y, 1 − z] for each oxygen atom. In summary, the intermolecular contacts result in a columnar arrangement of the molecules parallel to the a axis (Fig. 5 ▸).
Figure 4.
Predominant O⋯H—C contacts (blue dotted lines) of O atoms with the methyl H atoms of the acetylacetonate groups of neighbouring molecules. The central molecule is drawn in space-filling mode, while neighbouring molecules are drawn in the stick-model mode visualizing the delocalized π system of the acetylacetonate ligands.
Figure 5.
Columnar arrangement of the molecules along the a axis.
Synthesis and crystallization
The synthesis of the title compound by refluxing a suspension of dimethyltin oxide, Me2SnO, in acetylacetone, acacH, for several hours followed the procedure and experimental details described by McGrady & Tobias (1965 ▸). Single crystals for X-ray diffraction were grown from toluene solution. A suitable single crystal was selected under a polarization microscope and mounted on a 50 μm MicroMesh MiTeGen MicromountTM using FOMBLIN Y perfluoropolyether (LVAC 16/6, Aldrich). The crystals are stable in air.
Spectroscopic data: 1H NMR [CDCl3, TMS, δ (ppm)], nJ [Hz]): δ(CH3—Sn) = 0.58, 2 J(1H—119/117Sn) = 100.6/97.1; δ(CH3)acac = 1.96; δ(CH)acac = 5.31 (Lockhart & Manders, 1986 ▸; Otera et al., 1981 ▸); 13C NMR [CDCl3, TMS, δ (ppm)], n J [Hz]): δ(CH3—Sn) = 7.75, 1 J(13C—119/117Sn = 973.7/930.4), δ(CH3)acac = 27.94, δ(CH)acac = 100.09, δ(C=O)acac = 190.75 (Lockhart & Manders, 1986 ▸, Otera et al., 1981 ▸); IR [ATR, ν (cm−1)]: 3010 w, 2920 w,1562 s, 1512 s, 1436 m, 1361 s,bd, 1256 m, 1203 m, 1015 m, 925 m, 803 m, 781 m, 655 m, 572 m, 552 m (McGrady & Tobias, 1965 ▸); Raman [ν (cm−1)]: 3092 w, 2999 w, 2920 s, 2708 w, 1574 w, 1427 w, 1366 m, 1263 m, 1206 m, 1194 m, 1021 w, 927 m, 668 m, 567 m, 512 s, 415 m, 220 m, 130 m 94 m, 68 m (McGrady & Tobias, 1965 ▸).
Refinement details
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. All H atoms were clearly identified in difference Fourier syntheses but were refined assuming idealized geometries and allowed to ride on the carbon atoms with 0.98 Å (–CH3), and 0.95 Å (–CH–) and with U iso(H) = 1.2 and 1.5U eq(C), respectively.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Sn(CH3)2(C5H7O2)2] |
| M r | 346.97 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 100 |
| a, b, c (Å) | 6.7693 (4), 13.8357 (7), 7.6661 (4) |
| β (°) | 104.709 (2) |
| V (Å3) | 694.46 (7) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 1.84 |
| Crystal size (mm) | 0.29 × 0.23 × 0.16 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2009 ▸) |
| T min, T max | 0.616, 0.760 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 21644, 1672, 1456 |
| R int | 0.024 |
| (sin θ/λ)max (Å−1) | 0.660 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.013, 0.034, 1.11 |
| No. of reflections | 1672 |
| No. of parameters | 84 |
| Δρmax, Δρmin (e Å−3) | 0.37, −0.27 |
Supplementary Material
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S2056989017003206/wm5370sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017003206/wm5370Isup2.hkl
CCDC reference: 1534819
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank the Deutsche Forschungsgemeinschaft and the Government of Lower-Saxony for funding the diffractometer and acknowledge support by Deutsche Forschungsgemeinschaft (DFG) and Open Access Publishing Fund of Osnabrück University.
supplementary crystallographic information
Crystal data
| [Sn(CH3)2(C5H7O2)2] | F(000) = 348 |
| Mr = 346.97 | Dx = 1.659 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.7693 (4) Å | Cell parameters from 9878 reflections |
| b = 13.8357 (7) Å | θ = 2.9–28.5° |
| c = 7.6661 (4) Å | µ = 1.84 mm−1 |
| β = 104.709 (2)° | T = 100 K |
| V = 694.46 (7) Å3 | Prism, colourless |
| Z = 2 | 0.29 × 0.23 × 0.16 mm |
Data collection
| Bruker APEXII CCD diffractometer | 1456 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.024 |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | θmax = 28.0°, θmin = 3.0° |
| Tmin = 0.616, Tmax = 0.760 | h = −8→8 |
| 21644 measured reflections | k = −18→18 |
| 1672 independent reflections | l = −10→10 |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.013 | H-atom parameters not defined? |
| wR(F2) = 0.034 | w = 1/[σ2(Fo2) + (0.0135P)2 + 0.383P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.11 | (Δ/σ)max = 0.001 |
| 1672 reflections | Δρmax = 0.37 e Å−3 |
| 84 parameters | Δρmin = −0.27 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Sn1 | 0.5000 | 0.5000 | 0.0000 | 0.01302 (5) | |
| O1 | 0.64915 (18) | 0.48470 (8) | 0.28616 (14) | 0.0219 (2) | |
| C1 | 0.7415 (2) | 0.40942 (11) | 0.36115 (18) | 0.0190 (3) | |
| C2 | 0.7883 (2) | 0.32773 (11) | 0.2732 (2) | 0.0209 (3) | |
| H2 | 0.8493 | 0.2758 | 0.3489 | 0.055 (3)* | |
| C3 | 0.7571 (2) | 0.31231 (10) | 0.0877 (2) | 0.0166 (3) | |
| O2 | 0.66423 (16) | 0.36896 (7) | −0.03729 (13) | 0.0183 (2) | |
| C4 | 0.8070 (3) | 0.41338 (14) | 0.5638 (2) | 0.0314 (4) | |
| H41 | 0.8498 | 0.3489 | 0.6114 | 0.055 (3)* | |
| H42 | 0.6924 | 0.4353 | 0.6102 | 0.055 (3)* | |
| H43 | 0.9214 | 0.4585 | 0.6018 | 0.055 (3)* | |
| C5 | 0.8410 (3) | 0.22215 (11) | 0.0252 (2) | 0.0272 (3) | |
| H51 | 0.9046 | 0.1820 | 0.1298 | 0.055 (3)* | |
| H52 | 0.9435 | 0.2394 | −0.0397 | 0.055 (3)* | |
| H53 | 0.7300 | 0.1860 | −0.0553 | 0.055 (3)* | |
| C6 | 0.7456 (2) | 0.58584 (11) | −0.0348 (2) | 0.0236 (3) | |
| H61 | 0.8232 | 0.6103 | 0.0825 | 0.053 (4)* | |
| H62 | 0.6921 | 0.6403 | −0.1145 | 0.053 (4)* | |
| H63 | 0.8353 | 0.5466 | −0.0887 | 0.053 (4)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Sn1 | 0.01680 (7) | 0.01050 (7) | 0.01066 (7) | 0.00215 (5) | 0.00147 (5) | −0.00056 (5) |
| O1 | 0.0300 (6) | 0.0211 (6) | 0.0130 (5) | −0.0013 (4) | 0.0026 (4) | −0.0031 (4) |
| C1 | 0.0147 (6) | 0.0287 (8) | 0.0123 (6) | −0.0080 (6) | 0.0010 (5) | 0.0042 (6) |
| C2 | 0.0182 (7) | 0.0235 (8) | 0.0200 (7) | 0.0039 (6) | 0.0031 (6) | 0.0110 (6) |
| C3 | 0.0139 (6) | 0.0134 (6) | 0.0236 (7) | 0.0007 (5) | 0.0067 (5) | 0.0038 (6) |
| O2 | 0.0252 (5) | 0.0144 (5) | 0.0148 (5) | 0.0057 (4) | 0.0039 (4) | 0.0001 (4) |
| C4 | 0.0312 (8) | 0.0485 (11) | 0.0116 (7) | −0.0168 (8) | 0.0001 (6) | 0.0044 (7) |
| C5 | 0.0273 (8) | 0.0173 (7) | 0.0403 (10) | 0.0081 (6) | 0.0143 (7) | 0.0045 (7) |
| C6 | 0.0228 (7) | 0.0191 (7) | 0.0284 (8) | −0.0012 (6) | 0.0058 (6) | 0.0009 (6) |
Geometric parameters (Å, º)
| Sn1—C6 | 2.115 (2) | C3—C5 | 1.499 (2) |
| Sn1—C6i | 2.115 (2) | C1—C4 | 1.505 (2) |
| Sn1—O1 | 2.180 (1) | C4—H41 | 0.9800 |
| Sn1—O1i | 2.180 (1) | C4—H42 | 0.9800 |
| Sn1—O2 | 2.183 (1) | C4—H43 | 0.9800 |
| Sn1—O2i | 2.183 (1) | C5—H51 | 0.9800 |
| O1—C1 | 1.274 (2) | C5—H52 | 0.9800 |
| O2—C3 | 1.273 (2) | C5—H53 | 0.9800 |
| C1—C2 | 1.393 (2) | C6—H61 | 0.9800 |
| C2—C3 | 1.400 (2) | C6—H62 | 0.9800 |
| C2—H2 | 0.9500 | C6—H63 | 0.9800 |
| C6i—Sn1—C6 | 180.0 | O2—C3—C2 | 126.07 (13) |
| C6i—Sn1—O1i | 90.20 (5) | O2—C3—C5 | 115.27 (13) |
| C6—Sn1—O1i | 89.80 (5) | C2—C3—C5 | 118.66 (13) |
| C6i—Sn1—O1 | 89.80 (5) | C3—O2—Sn1 | 125.68 (9) |
| C6—Sn1—O1 | 90.20 (5) | C1—C4—H41 | 109.5 |
| O1i—Sn1—O1 | 180.0 | C1—C4—H42 | 109.5 |
| C6i—Sn1—O2 | 89.59 (5) | H41—C4—H42 | 109.5 |
| C6—Sn1—O2 | 90.41 (5) | C1—C4—H43 | 109.5 |
| O1i—Sn1—O2 | 94.01 (4) | H41—C4—H43 | 109.5 |
| O1—Sn1—O2 | 85.99 (4) | H42—C4—H43 | 109.5 |
| C6i—Sn1—O2i | 90.41 (5) | C3—C5—H51 | 109.5 |
| C6—Sn1—O2i | 89.59 (5) | C3—C5—H52 | 109.5 |
| O1i—Sn1—O2i | 85.99 (4) | H51—C5—H52 | 109.5 |
| O1—Sn1—O2i | 94.01 (4) | C3—C5—H53 | 109.5 |
| O2—Sn1—O2i | 180.0 | H51—C5—H53 | 109.5 |
| C1—O1—Sn1 | 125.61 (9) | H52—C5—H53 | 109.5 |
| O1—C1—C2 | 126.2 (1) | Sn1—C6—H61 | 109.5 |
| O1—C1—C4 | 114.7 (2) | Sn1—C6—H62 | 109.5 |
| C2—C1—C4 | 119.1 (1) | H61—C6—H62 | 109.5 |
| C1—C2—C3 | 128.4 (1) | Sn1—C6—H63 | 109.5 |
| C1—C2—H2 | 115.8 | H61—C6—H63 | 109.5 |
| C3—C2—H2 | 115.8 | H62—C6—H63 | 109.5 |
Symmetry code: (i) −x+1, −y+1, −z.
References
- Brandenburg, K. (2006). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Britton, D. (2006). Acta Cryst. C62, m93–m94. [DOI] [PubMed]
- Bruker (2009). APEX2, SADABS and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Lockhart, T. P. & Manders, W. F. (1986). Inorg. Chem. 25, 892–895.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- McGrady, M. M. & Tobias, R. S. (1965). J. Am. Chem. Soc. 87, 1909–1916.
- Miller, G. A. & Schlemper, E. O. (1973). Inorg. Chem. 12, 677–681.
- Otera, J., Hinoishi, T., Kawabe, Y. & Okawara, R. (1981). Chem. Lett. 10, 273–274.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S2056989017003206/wm5370sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017003206/wm5370Isup2.hkl
CCDC reference: 1534819
Additional supporting information: crystallographic information; 3D view; checkCIF report







