Abstract
Purpose of review
Because of the relatively small numbers of pediatric patients with congenital heart disease cared for in any individual center, there is a significant need for multicenter clinical studies to validate new medical or surgical therapies. The Pediatric Heart Network (PHN), with 15 years of experience in multicenter clinical research, has tackled numerous challenges when conducting multicenter studies.
Recent findings
This review describes the challenges encountered and the strategies employed to conduct high-quality, collaborative research in pediatric cardiovascular disease.
Summary
Sharing lessons learned from the PHN can provide guidance to investigators interested in conducting pediatric multicenter studies.
Keywords: congenital heart disease, pediatric cardiology, research
INTRODUCTION
Despite many advances and improvements, the care of children with heart disease still lacks a robust evidence base, and morbidity and mortality rates remain high. As compared to other pediatric sub-specialties, there are fewer pediatric cardiovascular trials registered in clinicaltrials.gov, and only a small proportion of these focus on congenital heart disease (CHD) [1■]. Because of the paucity of multicenter clinical studies in pediatric cardiovascular disease and the multiple rare conditions comprising CHD, the National Heart, Lung, and Blood Institute (NHLBI) established the Pediatric Heart Network (PHN) in 2001, a nimble infrastructure of multiple centers, designed to support a variety of clinical studies in pediatric cardiovascular disease.
The mission of the PHN is to improve health outcomes, disseminate collaborative findings, train and educate new investigators, and provide support and advocacy for families through the conduct of research. The PHN consists of nine main clinical sites, additional auxiliary sites, a data coordinating center, and the NHLBI (Fig. 1) [2]. The PHN has established best practices for multicenter research and has completed 11 studies, with an additional five underway and four nearing launch. These studies include phase I, II, and III clinical trials, observational studies, and quality improvement, nursing, and health services research studies (Table 1). In 2009, to encourage translation of research findings between the basic science, genetics and clinical arenas, the PHN was joined by the Cardiovascular Development Consortium and the Pediatric Cardiac Genomics Consortium; together these three programs make up the Bench to Bassinet translational research program.
FIGURE 1.
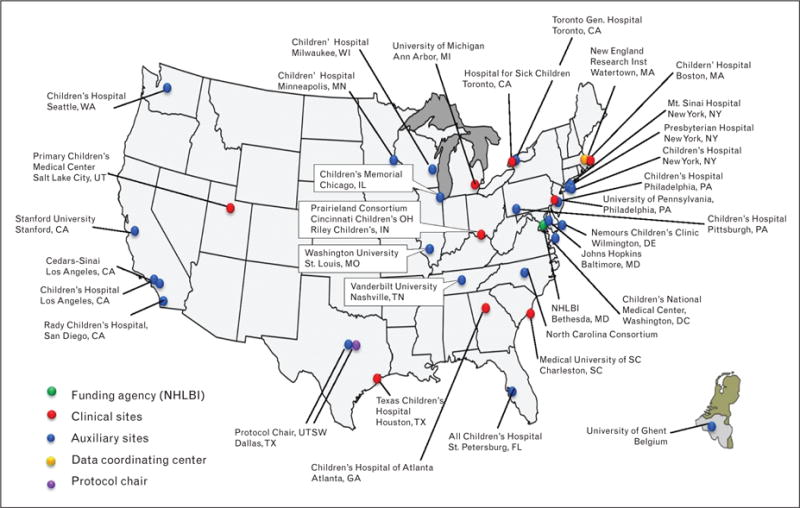
Pediatric Heart Network Centers. This figure illustrates the distribution of PHN centers throughout North America, including nine main centers, numerous auxiliary sites, a data coordinating center, and the NHLBI. Original figure.
Table 1.
Pediatric Heart Network studies
| Name | Yeara | Study design | Sample Size | Population | Sites | Primary endpoint(s) |
|---|---|---|---|---|---|---|
| Pulsed Corticosteroid Therapy for Primary Treatment of Kawasaki Disease | 2002–2004 | Phase III Clinical trial | 199 | Kawasaki disease | 8 | Coronary artery maximum Z-score |
| Fontan Cross-Sectional Study | 2003–2004 | Observational | 546 | Single ventricle | 7 | Quality of life, ventricular function, and performance |
| Fontan II (Follow-up of Fontan Study) | 2009–2011 | Observational | 428 | Single ventricle | 7 | Quality of life |
| Fontan III (Follow-up of Fontan II) | 2013–2014 | Observational | 373 | Single ventricle | 7 | Quality of life, ventricular function, and performance |
| Enalapril in Infants with Single Ventricle | 2003–2008 | Phase III Clinical trial | 230 | Single ventricle | 10 | Weight-for-age Z-score at 14 months |
| Single Ventricle Reconstruction (SVR) | 2005–2008 | Phase III Clinical trial | 555 | Single ventricle | 15 | Death/transplantation at 12 months |
| SVR II | 2008–2015 | Observational | 325 | Single ventricle | 15 | Death/transplantation at 6 years |
| SVR III | 2015–2020 | Observational | 264b | Single ventricle | 15 | Right ventricular function at 11 years |
| Ventricular Volume Variability | 2005–2007 | Observational | 131 | Dilated cardiomyopathy | 8 | Echocardiographic measures |
| Atenolol versus Losartan in Marfan Syndrome | 2007–2014 | Phase III Clinical trial | 608 | Marfan syndrome | 21 | Aortic root Z-score change |
| Training in Exercise Activities and Motion for Growth (TEAM 4 Growth) | 2013–2014 | Pilot: exercise intervention | 20 | Single ventricle | 3 | Safety and feasibility |
| Echo Z-Score | 2013–2015 | Development of normative data | 3600b | Normal children | 19 | Echocardiographic measures |
| ECG Z-Score | 2013–2015 | Development of normative data | 2700b | Normal children | 19 | Electrocardiographic measures |
| Collaborative Learning Project of Perioperative Care of Infants with Congenital Heart Disease | 2014–2015 | Pilot: collaborative learning Intervention | 579 | Tetralogy of Fallot, Coarctation of the aorta | 10 | Compliance with Clinical Practice Guideline for early extubation |
| Dexmedetomidine in Corrective Infant Cardiac Surgery | 2014–2016 | Phase I clinical study | 106b | d-Transposition, Tetralogy of Fallot | 3 | Safety and pharmacokinetics |
| Oxandrolone to Promote Growth in Infants with Hypoplastic Left Heart Syndrome | 2015–2018 | Phase I/II clinical study | 100b | Single ventricle | 9 | Safety and efficacy |
| Udenafil in Adolescents with Single Ventricle Physiology after Fontan | 2014 | Phase I/II clinical study | 36 | Single ventricle | 6 | Safety, pharmacokinetics, and efficacy |
| Fontan Udenafil Exercise Longitudinal Assessment (FUEL) | 2015–2017 | Phase III clinical trial | 300b | Single ventricle | 13 | Exercise performance |
| Validation of the Residual Lesion Score for Congenital Heart Surgery | 2015–2018 | Observational | 1250b | Five congenital heart malformations | 17 | Residual lesion score |
| Dyslipidemia of Obesity Intervention Trial (DO IT) | 2015–2018 | Phase III clinical trial (statin) | 354b | Obese adolescents | 13 | Vascular measures |
Year refers to the time period from beginning of enrolment through the end of scheduled follow-up.
Study not complete; number represents target enrolment.
The first challenge to multicenter collaboration is establishing an integrated infrastructure. Once the collaborative infrastructure is formed, the major challenges are sustaining the collaboration, asking the right scientific questions, ensuring study feasibility, recruiting and retaining study participants, overcoming regulatory hurdles, disseminating results, and remaining current.
Collaboration
The PHN operates on the ‘it takes a village’ principle, building on successful relationships within and outside of the PHN. Multidisciplinary research encourages unique study team configurations in which collaboration occurs among physicians, nurses, neurodevelopmental specialists, physical therapists, imaging experts, statisticians, and so on. The PHN is unique in that it provides financial, statistical, and mentoring support for nursing research, further expanding collaborations.
External collaborations are highlighted by the Marfan trial, which compared the effect of losartan and atenolol on aortic root outcomes [3,4■■]. The Marfan Foundation provided financial support and helped with recruitment and communication, using its prominence to encourage enrolment. This trial offers another example of external collaboration, in that the scientific ideas behind the trial were brought to the PHN by a non-PHN investigator. The PHN has also benefitted from collaborations with pharmaceutical companies which have donated study drugs and proposed partnerships with the PHN, with the goal of receiving pediatric labeling. Finally, the PHN has been fortunate to receive funds from the Food and Drug Administration’s (FDA) Office of Orphan Products Development and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. These internal and external collaborations have been instrumental in achieving our goals.
The PHN has been fortunate to have investigators who are committed to the mission and want it to succeed. Nevertheless, we made sure at the beginning to establish rules of engagement for potentially contentious activities. In particular, long before any articles were written, we established a publication process that would ensure appropriate recognition of effort and provide opportunities for as many people as possible, including study coordinators and junior faculty, to participate. We applied the same democratic principles to selecting auxiliary sites and ancillary studies. Our emphasis on collegiality has succeeded in fostering an environment in which investigators and coordinators rely upon one another for support and ideas and share lessons learned.
Asking the right scientific questions
Collaboration alone is not sufficient if investigators cannot identify and agree upon the most compelling scientific questions. The PHN takes a multi-pronged approach to this. First, both PHN and non-PHN investigators can propose ideas for studies. This open call for proposals permits us to harness the intellectual power of a broad range of expertise and to conduct studies of importance to the wider pediatric research community.
Second, the right scientific questions cannot always be answered by a clinical trial. Although the initial intent was for the PHN to conduct only clinical trials, it soon became apparent that key information, like anticipated effect size of various interventions, did not exist. The flexibility of the PHN infrastructure allows study design to adapt to the state of the science. For example, the Fontan study was originally intended to be a clinical trial but was ultimately executed as a prospective observational study because of lack of sufficient data to design a trial [5]. Other questions may benefit from a quality improvement approach, which is why we designed the Collaborative Learning Study, to determine whether creation and subsequent adoption of a clinical practice guide can improve patient outcomes such as earlier extubation following surgery.
Third, identifying the right scientific question requires a discussion of equipoise, a legitimate uncertainty as to which treatment is better. This has been a challenge for the PHN, as pediatric cardiology is often characterized by strongly-held, sometimes divergent beliefs. In the Single Ventricle Reconstruction trial [6,7], comparing two surgical procedures for initial palliation of single right ventricle, we knew that the surgeons’ willingness to randomize was key to the success of the trial. To assess equipoise, we obtained data on surgical practices and preferences at participating sites, and also asked all participating surgeons to affirm their commitment to the trial. As a result, we had no significant problems with recruitment. In the Infant Single Ventricle trial [8,9], in which enalapril was compared with placebo in infants after stage I palliation for single ventricle physiology, however, recruitment suffered by not assessing equipoise at the beginning of the study. Although the investigators who participated in protocol development had equipoise, the clinical providers caring for these infants often did not, resulting in significant unanticipated recruitment challenges [10].
Feasibility
Identifying the most important scientific question does not ensure that it is feasible to answer that question. The first step is to determine a clinically relevant endpoint. The FDA looks for endpoints that reflect how a patient survives, feels, or functions in daily life. In adult cardiovascular studies, endpoints include all-cause mortality or major adverse cardiac events (MACEs), such as myocardial infarction and stent thrombosis. Fortunately, individuals with CHD do not have high rates of mortality or MACE, but low rates of hard endpoints pose a problem for clinical trial design. Except for the Single Ventricle Reconstruction trial, which included a mortality endpoint, other PHN trials have all required surrogate endpoints. Mindful of the potential pitfalls of surrogate endpoints, we have focused on endpoints that are as clinically relevant as possible, using the following as our litmus test: would the trial results based on this endpoint influence practice? In all cases, we selected surrogate endpoints known to be linked to serious adverse outcomes, such as change in coronary artery diameter [11], somatic growth [9], and aortic root diameter [4■■].
After agreement on the endpoint, we need to determine the effect size based on differences between event rates in the study arms. This poses a particular challenge for the PHN because there is little published literature on likely changes in event rates as a result of one treatment or another. However, one of the advantages of the Network infrastructure is that it provides the resources to analyze data on event rates at individual sites, where these data may have been obtained for other reasons. In addition, our early studies have informed design of later trials. For example, the Fontan Udenafil Exercise Longitudinal Assessment (FUEL) study will test the use of a phosphodiesterase type 5 inhibitor in adolescents with Fontan physiology; results of the Fontan observational study were used to inform the design of the FUEL study.
Another aspect of feasibility is whether the PHN centers have sufficient numbers of eligible patients. A common pitfall is to simply count the number of patients with the requisite diagnosis, but this does not take into account the inclusion and exclusion criteria for a specific study. The Network infrastructure allows study coordinators to conduct searches of local databases and determine how many patients are eligible based on the actual trial protocol. We have emphasized this approach after learning a hard lesson from our attempt to conduct a trial of angiotensin-converting enzyme inhibition to treat mitral regurgitation in atrioventricular septal defects [12]. For this trial, we based the degree of mitral regurgitation after repair on published literature, which did not necessarily reflect current practice. This error led us to overestimate of the number of available patients with severe enough mitral regurgitation for inclusion in the study; the PHN ultimately shut down the trial for inability to recruit individuals. Although this was unfortunate, it does illustrate another advantage of the Network structure: it is less traumatic to shut down a Network study because there are other studies planned and ready to fill the void.
Recruitment and retention
Every clinical study struggles with recruitment and retention, and the PHN is no different. We have a series of strategies to help meet this challenge. We have the flexibility to add auxiliary sites when needed to augment the eligible population at the main PHN sites. This has not only helped with recruitment, but also has expanded the scientific talent pool. As a learning organization, we have adopted the approach of soliciting and sharing best practices across sites through conference calls and in-person meetings [10]. In addition, because the PHN is an ongoing enterprise, we can learn from each study and apply the information gained to subsequent studies. One strategy recently adopted is to require that recruitment and retention plans be established during protocol development. Finally, the Children and Clinical Studies Campaign (http://www.nhlbi.nih.gov/childrenandclinicalstudies/index.php), developed with PHN resources, offers patients and families an accessible means of learning about what it means to participate in clinical research, what they should consider before signing a consent form, and what questions they should ask.
The power of a study derives from both recruiting enough participants, and then retaining them through the protocol-specified follow-up period. We believe that the relationships established with the study staff, especially with the coordinators, help with study retention. Families can contact the coordinators at any time and obtain reassurance about any concerns they may have about study participation.
Regulatory hurdles
A common regulatory hurdle in clinical research is institutional review board (IRB) approval. One of the advantages of the PHN is that we have refined our protocol development over time and learned from our mistakes. At this point, IRBs are familiar with PHN protocols, leading to fewer delays in IRB review. The FDA has also become familiar with the PHN, which may help during the investigational new drug (IND) application process for our regulated trials.
A bigger challenge for the PHN, and for many other clinical researchers, is executing subcontracts with clinical sites and core laboratories. The PHN has recently implemented a master agreement with the main PHN sites, such that when additional subcontract language is required for the next study, the main concepts are already in place, and contract approval is facilitated. Pediatric clinical trials often require innovative strategies. In the Single Ventricle Reconstruction surgical trial, the standard approach to reporting serious adverse events used in drug trials was not working well because most of the standard perioperative care qualified as a serious adverse event. The study committee developed a ‘sentinel event’ approach to adverse event reporting [13], which ensured the safety of participants while decreasing the burden associated with reporting multiple adverse events in a very sick population. This approach was adopted in a subsequent NHLBI trial of hypothermia in children after cardiac arrest [14].
Dissemination of results
An important tenet of conducting clinical research is that the results be disseminated widely. A separate writing committee is formed for each PHN article, so that there are rotating groups of authors with opportunities for investigators from PHN and auxiliary sites, study coordinators, and trainees to participate. Because the funds for statistical analysis and article preparation are included in the PHN’s budget, there are fewer limits on the amount of valuable information that can be disseminated from our studies. PHN studies are published in a wide variety of journals that reach adult and pediatric cardiovascular audiences as well as general pediatricians.
An important component of disseminating findings is that this should occur in a timely fashion. NHLBI colleagues showed that a significant proportion of NHLBI-funded trials had not published their main results more than 2 years after the study was completed [15■]. The four PHN trials have been published an average of 16 months after the last patient visit; the recent Marfan trial main results article was published just 8 months after the last patient visit.
Remaining current
Since 2001, the PHN has evolved to meet changing scientific needs and opportunities as well as changing economic circumstances. The opportunity to collaborate with pharmaceutical companies has led to two new phase I/II trials, one of which expands PHN collaborations to include pediatric cardiac anesthesiologists. We have also begun two quality improvement studies. In the Collaborative Learning study, we are working with industrial engineers who help us understand complex workplace processes. In the Residual Lesion Score study, one of the aims is to compare the traditional process of data-gathering for clinical research (i.e., coordinators extracting data from charts) with the use of databases, registries, and electronic health records to gather the same data, in an attempt to see whether existing data sources can be used to streamline data acquisition in clinical research. In 2014, we established a Health Services and Outcomes Collaboratory, focused on opportunities for data integration across multiple existing databases, and the potential for conducting clinical trials based on registries, following the model of the adult cardiovascular trials Study of Access Site for Enhancement of Percutaneous Coronary Intervention in Women [16] or Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia [17]. The PHN has embraced the era of data sharing by making datasets from the Fontan study, the Single Ventricle Reconstruction, and Infant Single Ventricle trials available to a wider pool of investigators for secondary analysis on the PHN website (www.pediatricheartnetwork.com).
Part of remaining current is ensuring that the next generation of researchers is trained to help push us forward. PHN’s annual Career Day brings fellows and junior faculty from our main and auxiliary sites to participate in lectures and small-group discussions by PHN faculty and NHLBI staff on topics ranging from navigating National Institutes of Health (NIH) funding to designing a clinical trial. Since 2013, the PHN has also funded 6–7 PHN Scholars each year, who undergo rigorous peer review before being selected to receive financial support for a mentored project. The goal is to prepare them to submit a K23 NIH Mentored Award for Patient-Oriented Research or other funding application.
CONCLUSION
Multicenter clinical research brings challenges around every corner. Although each study has a learning curve, the PHN has adopted some key principles that help us work effectively and efficiently. The PHN is beginning to achieve its goal of influencing practice; PHN studies have been referenced in the guidelines for pediatric heart failure [18■] and adult CHD [19■]. The PHN will continue to evolve, in response to economic circumstances, to a hybrid model that includes industry partnerships and incorporates separate investigator-initiated funding for some clinical trials. As the PHN faces these new challenges, it will remain true to its principles of collaboration and continuous learning.
KEY POINTS.
The PHN has 15 years of experience with multicenter clinical research.
The challenges to multicenter collaboration are establishing an integrated infrastructure, sustaining the collaboration, asking the right scientific questions, ensuring study feasibility, recruiting and retaining study participants, overcoming regulatory hurdles, disseminating results, and remaining current.
As a learning organization, the PHN has evolved and developed strategies to meet these challenges.
Acknowledgments
None.
The comments expressed here are those of the authors, and do not reflect official positions of the National Heart, Lung, and Blood Institute or NIH.
Financial support and sponsorship
None.
Footnotes
Disclosures: no disclosures or conflicts of interest. This article has not been published in its current form or similar form, has not been accepted for publication elsewhere, and is not under consideration by another publication.
Conflicts of interest
No disclosures or conflicts of interest. The comments expressed here are those of the authors, and do not reflect official positions of the National Heart, Lung, and Blood Institute or NIH.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Hill KD, Chiswell K, Califf RM, et al. Characteristics of pediatric cardiovascular clinical trials registered on clinicaltrials. Gov Am Heart J. 2014;167:921–929 e922. doi: 10.1016/j.ahj.2014.02.002. This study examines the portfolio of clinical trials registered in clinicaltrials.gov. Of the 68,000 trials, only 5000 are pediatric trials, and only 284 were focused on pediatric cardiovascular interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahony L, Sleeper LA, Anderson PA, et al. The pediatric heart network: a primer for the conduct of multicenter studies in children with congenital and acquired heart disease. Pediatr Cardiol. 2006;27:191–198. doi: 10.1007/s00246-005-1151-9. [DOI] [PubMed] [Google Scholar]
- 3.Lacro RV, Dietz HC, Wruck LM, et al. Rationale and design of a randomized clinical trial of beta-blocker therapy (atenolol) versus angiotensin ii receptor blocker therapy (losartan) in individuals with marfan syndrome. Am Heart J. 2007;154:624–631. doi: 10.1016/j.ahj.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacro RV, Dietz HC, Sleeper LA, et al. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med. 2014;371:2061–2071. doi: 10.1056/NEJMoa1404731. This randomized clinical trial of 608 individuals 6 months–25 years of age with Marfan syndrome and aortic root dilation compared atenolol with losartan. After 3 years of treatment, there was no difference in the rate aortic root dilation between the groups. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sleeper LA, Anderson P, Hsu DT, et al. Design of a large cross-sectional study to facilitate future clinical trials in children with the fontan palliation. Am Heart J. 2006;152:427–433. doi: 10.1016/j.ahj.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohye RG, Gaynor JW, Ghanayem NS, et al. Design and rationale of a randomized trial comparing the blalock-taussig and right ventricle-pulmonary artery shunts in the norwood procedure. J Thorac Cardiovasc Surg. 2008;136:968–975. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu DT, Mital S, Ravishankar C, et al. Rationale and design of a trial of angiotensin-converting enzyme inhibition in infants with single ventricle. Am Heart J. 2009;157:37–45. doi: 10.1016/j.ahj.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu DT, Zak V, Mahony L, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122:333–340. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pike NA, Pemberton V, Allen K, et al. Challenges and successes of recruitment in the ‘angiotensin-converting enzyme inhibition in infants with single ventricle trial’ of the pediatric heart network. Cardiol Young. 2013;23:248–257. doi: 10.1017/S1047951112000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newburger JW, Sleeper LA, McCrindle BW, et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of kawasaki disease. N Engl J Med. 2007;356:663–675. doi: 10.1056/NEJMoa061235. [DOI] [PubMed] [Google Scholar]
- 12.Li JS, Colan SD, Sleeper LA, et al. Lessons learned from a pediatric clinical trial: the pediatric heart network angiotensin-converting enzyme inhibition in mitral regurgitation study. Am Heart J. 2011;161:233–240. doi: 10.1016/j.ahj.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virzi L, Pemberton V, Ohye RG, et al. Reporting adverse events in a surgical trial for complex congenital heart disease: the pediatric heart network experience. J Thorac Cardiovasc Surg. 2011;142:531–537. doi: 10.1016/j.jtcvs.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pemberton VL, Browning B, Webster A, et al. Therapeutic hypothermia after pediatric cardiac arrest trials: the vanguard phase experience and implications for other trials. Pediatr Crit Care Med. 2013;14:19–26. doi: 10.1097/PCC.0b013e31825b860b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon DJ, Lauer MS. Publication of trials funded by the national heart, lung, and blood institute. N Engl J Med. 2014;370:782. doi: 10.1056/NEJMc1315653. This study examined 244 NHLBI-funded randomized clinical trials completed between January 2000 and December 2011 and found that only 57% had published the main results within 30 months after completion of the trial. [DOI] [PubMed] [Google Scholar]
- 16.Hess CN, Rao SV, Kong DF, et al. Embedding a randomized clinical trial into an ongoing registry infrastructure: unique opportunities for efficiency in design of the study of access site for enhancement of percutaneous coronary intervention for women (safe-PCI for women) Am Heart J. 2013;166:421–428. doi: 10.1016/j.ahj.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frobert O, Lagerqvist B, Gudnason T, et al. Thrombus aspiration in st-elevation myocardial infarction in scandinavia (taste trial). A multicenter, prospective, randomized, controlled clinical registry trial based on the swedish angiography and angioplasty registry (scaar) platform. Study design and rationale. Am Heart J. 2010;160:1042–1048. doi: 10.1016/j.ahj.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 18.Kirk R, Dipchand AI, Rosenthal DN, et al. The international society of heart and lung transplantation guidelines for the management of pediatric heart failure: executive summary. J Heart Lung Transplant. 2014;33:888–909. doi: 10.1016/j.healun.2014.06.002. This guideline document summarizes the recommendations for clinical management of children with heart failure. [DOI] [PubMed] [Google Scholar]
- 19.Bhatt AB, Foster E, Kuehl K, et al. Congenital heart disease in the older adult: a scientific statement from the american heart association. Circulation. 2015;131:1884–1931. doi: 10.1161/CIR.0000000000000204. This scientific statement provides consensus guidelines for the management of adults with CHD. [DOI] [PubMed] [Google Scholar]


