Fig. 6. GCN5 and PCAF account for CD8+ T cell-mediated Rae-1 restoration.
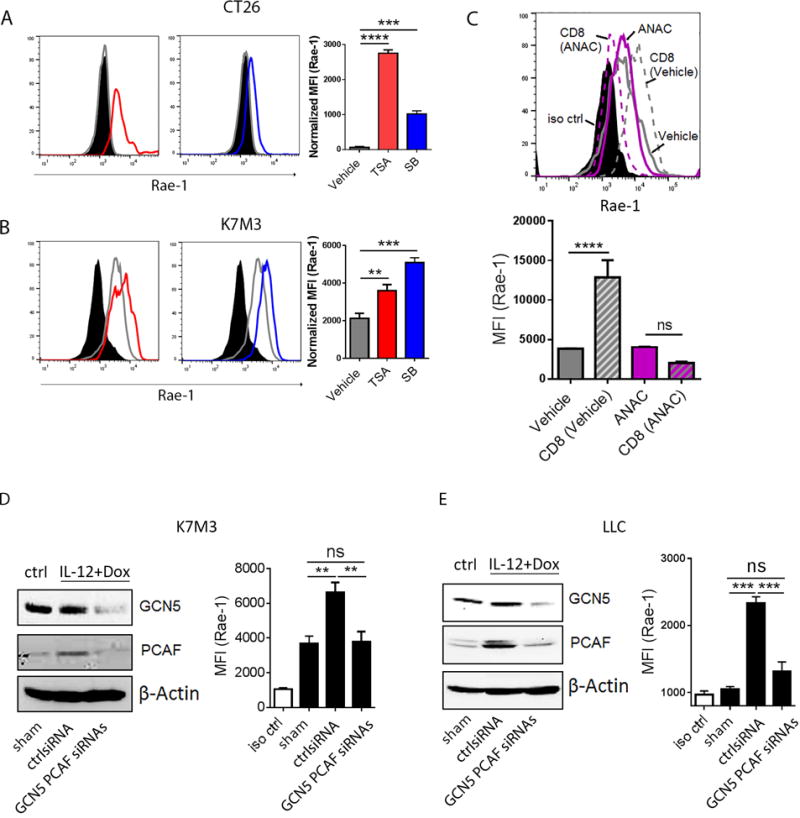
(A, B) HDAC inhibitors induced Rae-1 on CT26 and K7M3 tumor cells. CT26 (A) and K7M3 (B) cells were treated with vehicle control, TSA (0.3 μM) or SB (1 mM) for 24 hours. Rae-1 was detected by using flow cytometry. Black filled, isotype control; grey line, vehicle control; red line, TSA treated; blue line, SB treated. (C) HAT inhibitor anacardic acid (ANAC) abolished CD8+ T cell mediated Rae-1 restoration. K7M3 cells were pre-treated with vehicle control or ANAC (15 μM) for 3 hours, and subsequently co-incubated with CD8+ T cells at a ratio of 1:1 for 24 hours. Rae-1 expression on tumor cells was determined by flow cytometry. Black filled, isotype control; grey line, vehicle control; grey dash, vehicle and CD8+ T cells; purple line, ANAC; purple dash, ANAC and CD8+ T cells. (D, E) Loss of GCN5 and PCAF abrogated CD8+ T cell mediated Rae-1 restoration. K7M3 (D) and LLC (E) tumor-bearing mice (n = 5) were subjected to intratumoral administrations of control siRNA or GCN5 and PCAF siRNAs plus electroporation twice weekly, starting on day 7 after tumor inoculation. All the mice were treated with IL12 plus doxorubicin as described in Figs. 1 and 2. Four days after the second treatment, tumors were dissociated to test GCN5 and PCAF by immunoblots, and Rae-1 by flow cytometry. Bar graphs show the mean fluorescence intensity (MFI) of Rae-1 as means ± SEM. Results are representative of three repeated experiments.
