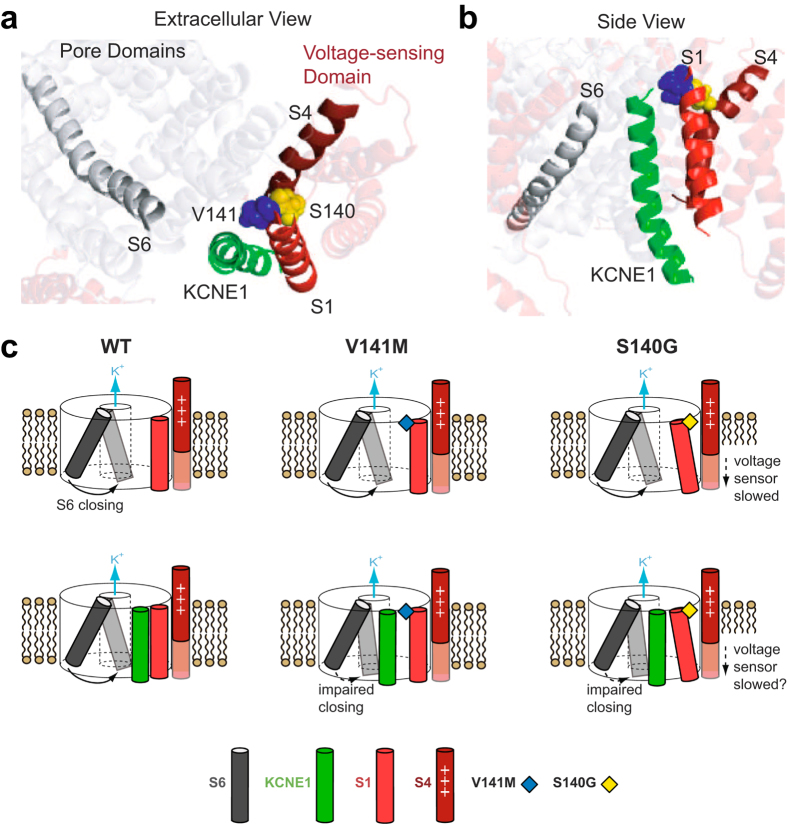
Figure 8. Proposed molecular mechanisms underlying effects of S140G and V141M on channel gating.
(a,b) Homology model of open tetrameric KCNQ1 and KCNE1 from Kang et al.12 from an extracellular (a) or side view (b). On the S1 helix, S140 (yellow) points toward S4, whereas V141 (blue) points toward KCNE1. The extracellular end of the S6 helix is in proximity to KCNE1. (c) Cartoon representation of proposed molecular mechanisms of S140G and V141M. The channel pore is represented by a cylinder with the K+ permeation pathway at its center. For KCNQ1 alone (top row), V141M has minimal effect on channel gating, whereas S140G disrupts S4 and slows its movement. In the presence of KCNE1 (bottom row), both V141M and S140G alter VSD-pore coupling and slow pore closing. V141M may directly disrupt the orientation of KCNE1, impairing motion of the S6 during channel closing. S140G may cause a similar disruption of KCNE1 indirectly through other residues on S1 that face KCNE1.