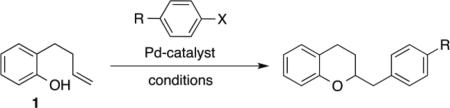
Table 3.
Comparison of Conditions for Chroman Synthesisa
Reaction Conditions for X = OTf: 1.0 equiv 1, 1.2 equiv R-OTf, 1.4 equiv LiOtBu, 2 mol % Pd(OAc)2, 5 mol % ligand, solvent (0.125 M), 98 °C, 16 h. Reactn Conditions for X = Br: 1.0 equiv 1, 2.0 equiv R–Br, 2.0 equiv NaOtBu, 2 mol % Pd2(dba)3, 4 mol % S-Phos, toluene (0.25 M), 110 °C.
Yields are isolated yields (average of two or more experiments) of material with >95% purity.
Yields as reported in reference 12.