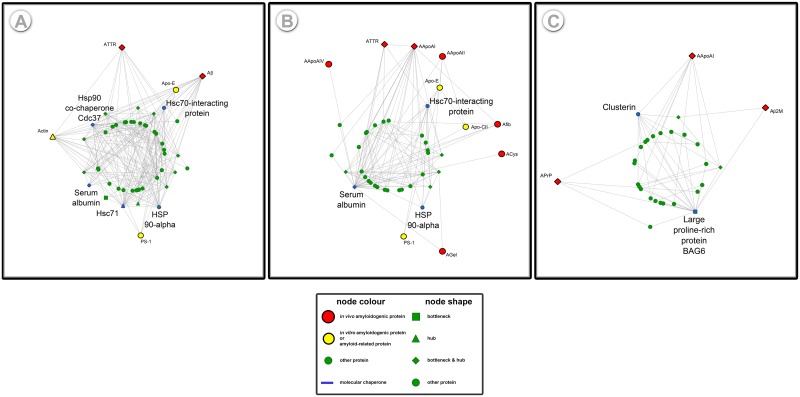
Fig 3. Subnetworks of molecular chaperones participating in the amyloid interactome.
3 important subnetworks were isolated from the entire amyloid interactome: (A) Subnetwork of Hsp90 co-chaperone Cdc37, Hsc70-interacting protein, Hsp 90-alpha, Hsc71 and their first neighbors, (B) Subnetwork of Serum albumin and Hsc70-interacting protein and their first neighbors and (C) Subnetwork of Clusterin, Large proline-rich protein BAG6 and their first neighbors. The aforementioned proteins, having chaperone or co-chaperone activity, were found to play a pivotal role in the integrity of the interactome (See section Network Analysis Based on Graph Theory). A highly selective and direct correlation of Serum albumin and 6 amyloidogenic proteins was observed (B), whereas indirect interactions between Serum albumin and 2 amyloidogenic proteins were recorded (A). Hsc70-interacting protein is a significant element of the interactome, since it conciliates interactions between Apolipoproteins and ACys or ATTR (A,B). Clusterin synergistically with Large proline-rich protein BAG6 interferes with APrp and Aβ2M (C). The finding that more than one chaperones mediate the interconnection between different amyloidogenic proteins deserves further investigation.