Abstract
Unlike organized lymphoid tissue, the tumor microenvironment (TME) often includes a high proportion of immunosuppressive macrophages. We model the TME by culturing peritoneal cavity (PerC) cells that naturally have a high macrophage to lymphocyte ratio. Prior studies revealed that, following TCR ligation, PerC T cell proliferation is suppressed due to IFNγ-triggered inducible nitric oxide synthase expression. In this study we assessed the ability of PerC B cells to respond to surrogate activating signals in the presence of high numbers of macrophages. Surface IgM (BCR) ligation led to cyclooxygenase-mediated, and TLR-4 ligation to IL10-mediated, suppression of PerC B cell proliferation. In contrast, PerC B cells had a robust response to CD40 ligation, which could overcome the suppression generated by the BCR or TLR-4 response. However, the CD40 response was suppressed by concurrent TCR ligation. These results reveal the challenges of promoting B and T cell responses in macrophage-rich conditions that model the TME.
Keywords: B cell, CD40, macrophage, tumor microenvironment
1. Introduction
Lymphoid organs harbor white blood cell subsets apportioned in a manner, e.g., follicles, marginal zone, etc., that facilitates surveillance of lymphatic fluid, blood, and mucosal surfaces. A general feature of all such tissues is a high lymphoid (B and T lymphocytes) to myeloid, (macrophages, dendritic cells) ratio that is essential for initiating immunity. In contrast, the leucocyte distribution found in many forms of neoplasia, e.g., carcinomas of the breast, colon, or ovaries, is distinctive for having an increased representation of immunosuppressive myeloid cells [1–3]. Immunity within such tumor microenvironments (TMEs) is often constrained and altered myeloid/lymphoid ratios have been found prognostic of further cancer development [2,4].
Considerable effort has been invested in defining how the cellular and molecular composition of the TME tempers T cell function. This work has revealed a role for regulatory receptor-ligand pairs (PD1/PDL1, CTLA-4/B7, etc), cytokines (TGF-β, IL-1, IL-6, IL-10, IL-17, etc), enzymes (iNOS, ARG, IDO, COX, etc.), and subsets of T (Tregs, TH17) and myeloid cells (TAMs, MDSCs, etc) in the suppression of anti-tumor T cell function [5–9]. These efforts have informed novel clinical approaches and fostered an exciting era of T cell-focused immunotherapies [5,8]. Myeloid cells, essential for many of the immunosuppressive elements found within the TME, are key targets in current immunotherapeutic drug discovery [8]. Focus upon T cells and regulatory APCs is logical as their interaction is central to the initiation of adaptive anti-tumor immunity. In contrast, B lymphocytes within TMEs, functioning as humoral or APC effectors, or as regulators of cell-mediated immunity, have been less studied [10].
Pliable, in vitro models that mimic the TME are essential to dissect the complex cellular and molecular interactions that lead to compromised anti-tumor immunity. We have shown that peritoneal cavity (PerC) T cell proliferation is suppressed by the increased representation of macrophages present in this cell source [11]. T cell proliferation is regulated via catabolism of limiting amino acids, production of anti-inflammatory cytokines, and prostaglandin production [11–14]. PerC cell preparations include a significant fraction of B cells, notably the B1 subset distinctive for antibody responses to commensal microflora [15]. B1 cells, like the more recently described B10 or Breg subset, are also distinctive for autocrine IL-10 production, a cytokine that antagonizes cytotoxic T lymphocyte (CTL) generation [15,16]. TMEs have been reported to include B cells with this and other immunosuppressive (TGF-β production, PD1/PDL1 expression) characteristics [10,17–20].
In this report we characterize PerC B cell susceptibility to macrophage suppression and show that the agent used to activate B cells dictates their response. The PerC B cell response to BCR ligation was suppressed by cyclooxygenase (COX) activation and to LPS stimulation by IL-10 production. In contrast, CD40 ligation triggered PerC B cell proliferation regardless of macrophage density. However, this response could be suppressed by concurrent activation of PerC T cells. This hierarchy of lymphoid suppression is discussed in the context of strategies to revitalize adaptive immunity within macrophage-rich TMEs.
2. Materials and Methods
2.1 Mice
Two to four month old male and female mice, bred and maintained at Rider University, were handled in accord with NIH, Animal Welfare Act, and Rider University IACUC guidelines. Breeding pairs of C57BL/6J, BALB/c, CB17-Prkdcscid/J, IFNγR−/− (B6.129S7Ifngr/J), iNOS−/− (B6.129P2-Nos2tm1Lau/J), and IL4−/− (B6.129P2-IL4tm1Cgn/J) mice were obtained from the Jackson Laboratory, Bar Harbor, ME.
2.2 Preparation of cell suspensions and cell culture
Spleen (SP) cell suspensions were obtained by gentle disruption of the organ between the frosted ends of sterile glass slides. Red blood cells were removed from SP cell preparations by hypertonic lysis followed by washing with Hanks Balanced Salt Solution (HBSS) (Life Technologies, Grand Island, NY). Peritoneal cavity (PerC) cells were obtained by flushing the peritoneum with 10 mls of warm (37 °C) HBSS supplemented with 2–3% fetal bovine serum (FBS) (Hyclone, Logan, UT). Viable cell counts were determined by Trypan blue exclusion. Various dilutions (1.0 – 4.0 × 106/ml) of cells, in RPMI 1640 culture media (Life Technologies) supplemented with 10% FBS (Hyclone), 0.1 mM nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin, 2 mM L-glutamine, 2 × 10−5 M 2-ME, and 10 mM HEPES, were plated in 96-well “V”- or flat-bottom microtiter plates (Corning Costar, Fisher Scientific, Pittsburgh, PA) and then incubated in a humidified atmosphere of 5% CO2 at 37 °C for 48 hrs. Most experiments plated cells at 3 x 106/ml in a V-bottom plate unless otherwise specified. For anti-CD3 stimulation soluble anti-CD3ε monoclonal antibody (mAb) (clone 145-2C11) (eBioscience, San Diego, CA) was added at 1.0 μg/ml. For anti-IgM stimulation F(ab′)2 fragment of goat anti-mouse IgM, μ-chain-specific (Jackson ImmunoResearch, West Grove, PA) polyclonal antibody was added at 10 μg/ml. LPS from E. coli, serotype R515 (Enzo Life Sciences, Farmingdale, NY or Sigma) was added at 5 μg/ml. Concanavalin A from Canavalia ensiformis (Sigma) was added at 1.0 μg/ml. Anti-CD40 mAb (clone FGK45) (Enzo) was added at 2.0 μg/ml. To inhibit arginine catabolism, the arginase (ARG) inhibitor N-ω-hydroxy-nor-L-arginine (1-NA; CalBiochem, San Diego, CA) or the inducible nitric oxide synthase (iNOS) inhibitor NG-monomethyl-L-arginine (1-MA; CalBiochem) were added. To inhibit tryptophan catabolism via indoleamine-2,3-dioxygenase (IDO), 1-methyl-tryptophan (1-MT; CalBiochem) was added. To inhibit COX, indomethacin (INDO; Sigma) was added. Neutralizing anti-mouse mAb for IFNγ (clone XMG1.2), IL-10 (clone JES5-16E3), or IL-4 (clone 11B11) were added at 7.5 μg/ml (eBioscience). Exogenous, recombinant murine IL-4, IL-10, and IL-13 cytokines were added at 10 ng/ml (Peprotech, Rocky Hill, NJ). All inhibitors, cytokines, or neutralizing MAbs were added at culture initiation. Optimal concentrations of all reagents were determined in titration experiments. After 44 hours, 1 μCi of [3H] thymidine (Moravek Inc., Brea, CA) was added to each well. The plates were frozen 4 hours after labeling, and then thawed for harvesting onto filter paper mats using a semi-automated cell harvester (Skatron Instruments, Richmond, VA). Radioactivity was measured by liquid scintillation spectrometry. For each experiment 5 wells were established for each test group. All experiments were done a minimum of 3 times, the majority more than 5 times.
2.3 Immunofluorescence staining and flow cytometric analyses
PerC and SP cell suspensions were first blocked with a “blocktail” of rat anti-mouse CD16/32 MAb (Fc Block, eBioscience) and 2% normal rat serum (Jackson ImmunoResearch, West Grove, PA). Cell suspensions were then stained using titered amounts of FITC-, Cy-Chrome-, or PE-labeled rat anti-mouse CD4, CD8, IgM, CD11b, CD45R, CD5 and/or F4/80 mAbs (eBioscience). Isotype- and fluorochrome-matched, nonspecific mAb controls were employed to establish analysis gates. For carboxyfluorescein succinimidyl ester (CFSE) cell proliferation assays cells were labeled with CellTrace CFSE Cell Proliferation Kit as described by the manufacturer (Thermo Fisher, Eugene, OR) prior to culture. The percentage of lymphocytes or myeloid cells co-expressing sets of these markers were determined via multiparameter flow cytometric analyses on a FACSCalibur™ flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) by FSC/SSC gating of the lymphoid or myeloid population using CellQuest software.
2.4 Statistical Analyses and Stimulation Index (SI)
T cell proliferative responses are presented as the average CPM (counts per minute) ± SEM (standard error of the mean). Data sets were compared using the Student’s t-test with p-values below 0.05 considered statistically significant: * = p < 0.05, ** = p < 0.005, *** = p < 0.0005 relative to control. The stimulation index (SI) is defined as the average CPM for the drug response (e.g., anti-IgM + IL-4) divided by the average CPM for the appropriate control response (anti-IgM alone).
3. Results
3.1 Peritoneal macrophages suppress B cells activated by BCR or TLR-4 ligation
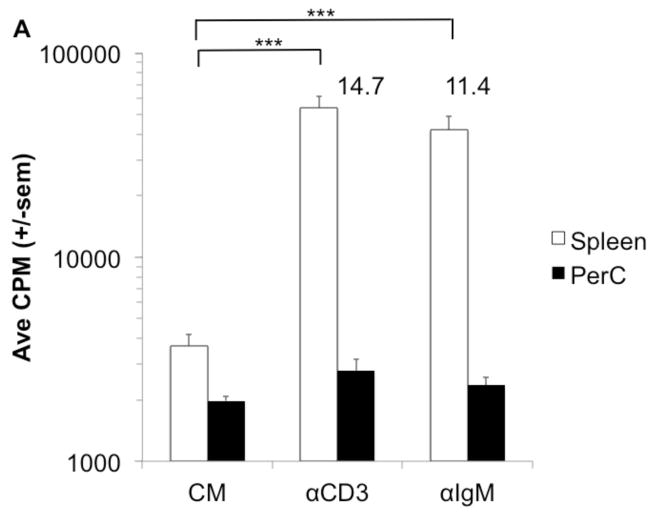
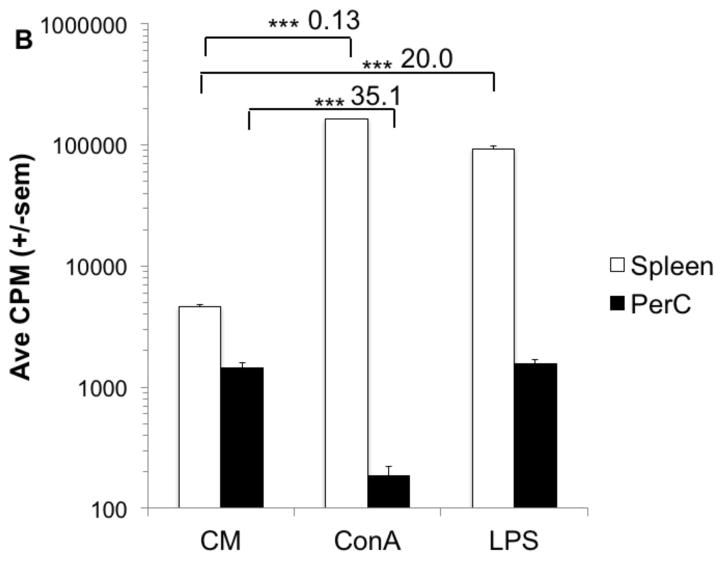
Our prior studies of peritoneal cavity (PerC) leucocyte biology focused on how macrophages (Mϕs) regulate the T lymphocyte response [11,12]. Peritoneal resident CD11bhi, F4/80+ Mϕs were found to temper the T cell response to either TCR ligation with anti-CD3 MAb or to the mitogen ConA (Figs. 1A, B). In this report we focused upon macrophage regulation of the PerC B cell response and found that both BCR (F(ab′)2 anti-IgM) and TLR-4 (LPS) ligation induced negligible B cell proliferation (Figs. 1A, B). These results contrast the significant proliferative response of spleen (SP) T and B cells to the same stimulation sources (Figs. 1A, B).
Figure 1. T and B cell proliferation is suppressed in PerC cell cultures.
C57BL/6J PerC and SP cells were cultured with anti-TCR or -BCR MAbs (αCD3, F(ab′2) αIgM) (A) or B or T cell mitogens (LPS, ConA) (B). * = p < 0.05, ** = p < 0.005, *** = p < 0.0005 relative to control. Numbers depict stimulation indices (SI) and represent the experimental CPM value divided by the pertinent control (complete media (CM) or stimulation alone).
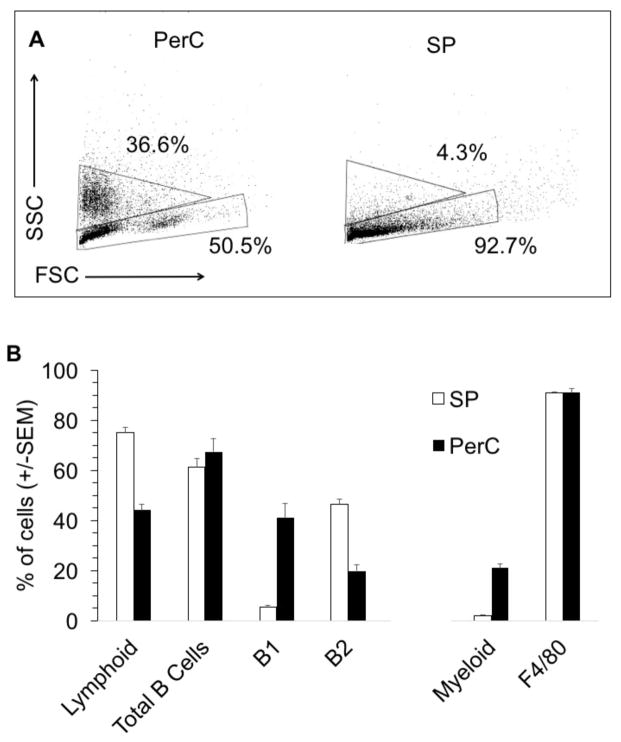
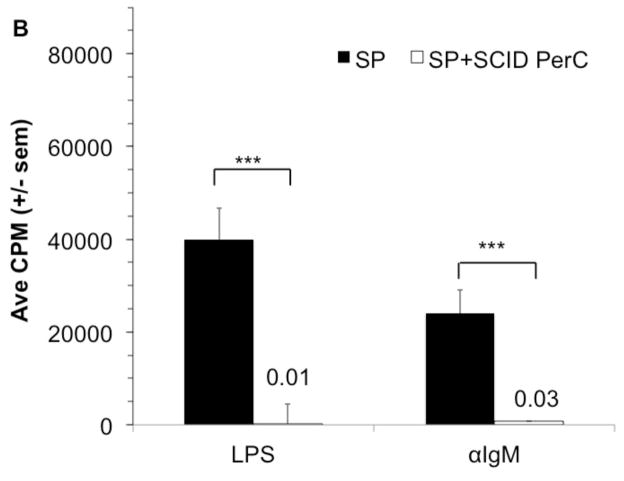
SP and PerC cell preparations differ in both their lymphoid and myeloid compartments (Fig. 2A, B). In terms of lymphoid composition, there are considerably more B-1 B cells in the PerC. Although F4/80+ macrophages comprise the majority of the myeloid fraction for both tissue sources, the greater representation of myeloid cells in the PerC leads to cultures that parallel tumor microenvironments rather than organized lymphoid tissue. Spleen T and B lymphocyte proliferation is suppressed when SP cells are co-cultured with PerC cells (Fig 3A). This regulation is due to Mϕs since PerC cells from SCID mice, which lack mature lymphocytes, suppressed both SP T and B cell responses (Fig 3B). These results illustrate that resident PerC Mϕs have the capacity to suppress both B and T cell proliferation.
Figure 2. Greater myeloid composition of PerC cells.
FSC and SSC profiles of PerC and SP cells from C57BL/6J mice (A). Numbers reflect the percentage of myeloid (upper gate) and lymphoid (lower gate) cells and are representative of compiled data illustrated in panel B. Percentages of lymphoid cells, total B cells (IgM+), SP B1 (IgM+, CD5+) and B2 B (IgM+, CD5−) cells, PerC B1 (IgM+, CD11b+) and B2 (IgM+, CD11b−) cells, and percentages of myeloid cells, predominately macrophages (F4/80+, CD11b+), in C57BL/6J mice (B).
Figure 3. PerC macrophages suppress spleen T and B cell responses.
C57BL/6J SP cells at 2 x 105 cells/well were cultured with αCD3, αIgM, or LPS alone or with PerC cells at 1 x 105 cells/well (A). BALB/c SP cells were cultured with LPS or αIgM alone or with CB.17 SCID PerC cells (B).
3.2 Peritoneal macrophages suppress B cells by prostaglandins and IL-10
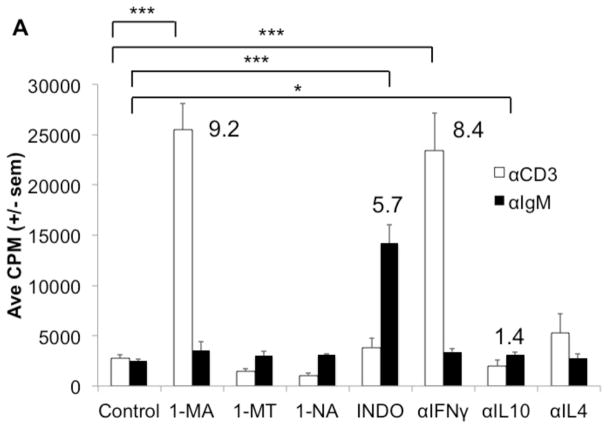
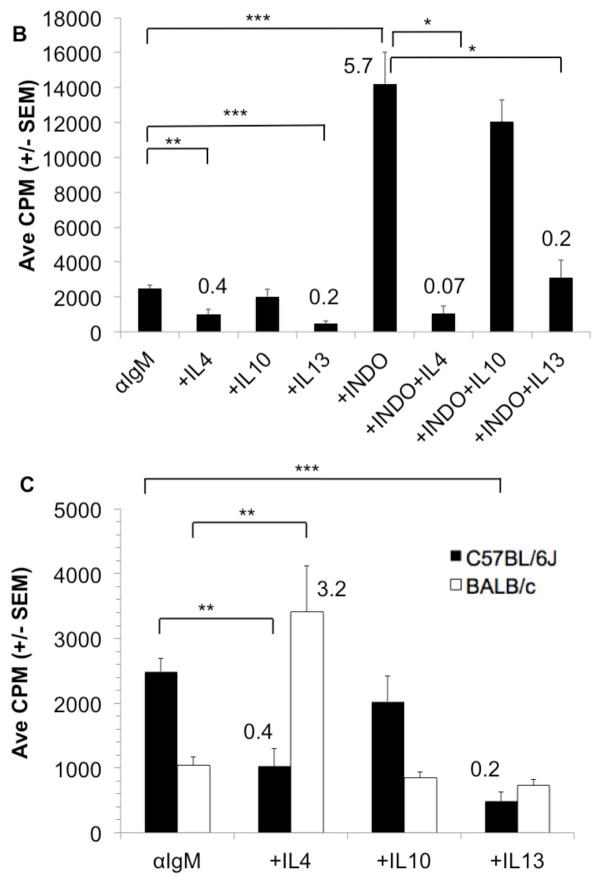
C57BL/6J PerC Mϕs temper TCR-triggered activation by inducing IFNγ-driven iNOS expression, which promotes proliferative arrest via arginine catabolism [11,12,14]. Inhibition of iNOS with 1-MA or neutralization of IFNγ abrogated PerC T cell suppression (Fig. 4A). To determine how Mϕs regulate PerC B cell activation in C57BL/6J mice, inhibitors of enzymes (iNOS/1-MA, IDO/1-MT, ARG/1-NA, COX/INDO), neutralizing MAbs for key regulatory cytokines (IFNγ, IL10, IL4) and addition of exogenous cytokines (IL-4, IL-10, IL-13) associated with immune regulation were tested. The suppressed response to BCR ligation was best recovered by inhibition of COX with indomethacin (INDO, Fig. 4A). Addition of IL-4 and IL-13 further suppressed C57BL/6J B cell proliferation and reduced the response released by COX inhibition (Fig 4B). In contrast, IL-4 helped the BALB/c BCR response whereas IL-10 and IL-13 had no effect (Fig 4C). These differences in C57BL/6J (TH1) versus BALB/c (TH2) mice PerC B cell responses reflect the TH1/TH2 profiles previously noted for their PerC T cells [12].
Figure 4. Regulation of the PerC B cell response to BCR ligation by exogenous cytokines or prostaglandin.
C57BL/6J PerC cells were cultured with αCD3 or αIgM +/− the inducible nitric oxide synthase (iNOS) inhibitor NG-monomethyl_L-arginine (1-MA), the indoleamine 2,3 dioxygenase inhibitor (IDO) 1 methyl-tryptophan (1-MT), the arginase (ARG) inhibitor N-ω-hydroxy-nor L arginine (1-NA), the cyclooxygenase (COX) inhibitor indomethacin (INDO), αIFNγ, αIL-10, and/or αIL-4 (A). The exogenous cytokines IL-4, IL-10, or IL-13 +/− indomethacin were added to C57BL/6J PerC cells cultured with αIgM (B). C57BL/6J and BALB/c PerC cells were cultured with αIgM and exogenous cytokines (C).
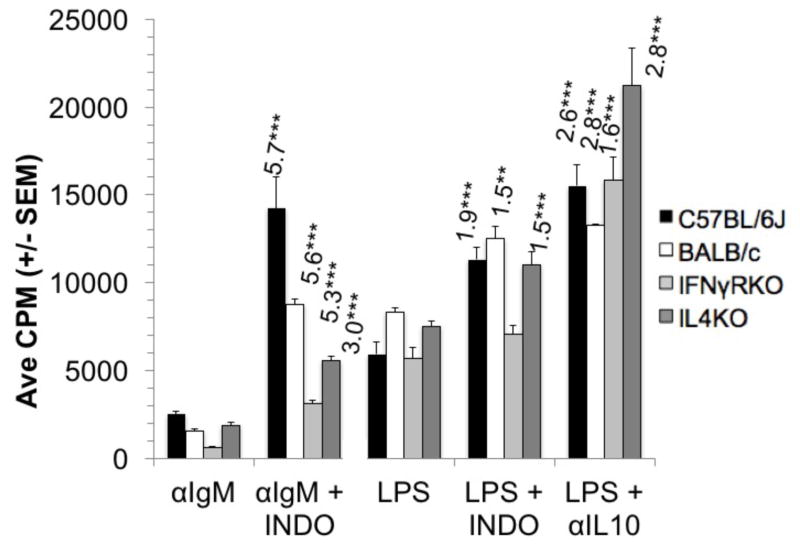
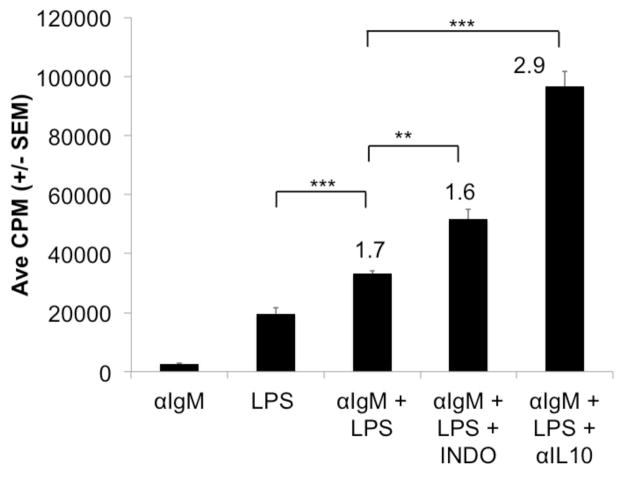
As noted for PerC cells from both C57BL/6J and BALB/c mice, the response to BCR ligation was suppressed in both IFNγR−/− and IL-4−/− cells and released by COX inhibition (Fig. 5). Likewise, the LPS response was suppressed with all PerC cell types tested; neutralization of IL-10 released this response in all strains. COX inhibition modestly improved the LPS response for all cell types excepting those from IFNγR−/− mice. In addition, the response to combined stimulation by BCR and TLR-4 ligation is greater than that of either form of stimulation alone (Fig. 6). Still, the response to dual stimulation remains tempered by COX and IL-10, neutralization of IL-10 provides the greatest response. These results illustrate that macrophages, via prostaglandins and IL-10, regulatory molecules found in TMEs, have the ability to temper B cell activation.
Figure 5. PerC B cell responses to BCR ligation or LPS.
PerC cells from C57BL/6J, BALB/c, IFNγR−/−, and IL-4−/− mice were activated with αIgM or LPS +/− IL-10 or indomethacin.
Figure 6. PerC B cell responses to concurrent BCR and TLR-4 ligation.
C57BL/6J PerC cells were cultured with αIgM, LPS, or αIgM + LPS +/− INDO or αIL-10.
3.3 Differences in B cell subset responses during macrophage regulation
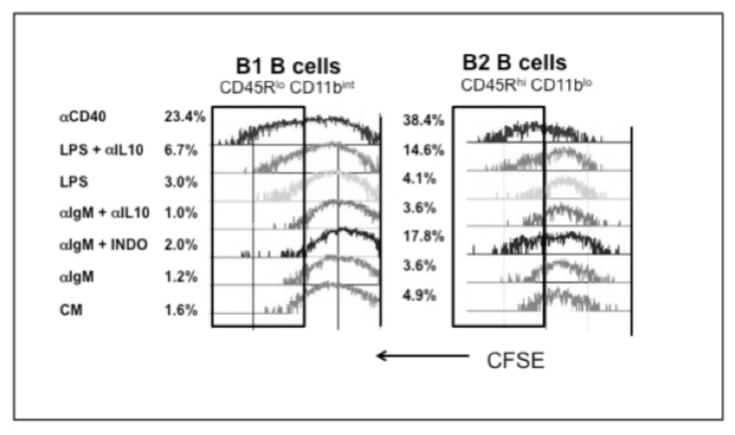
Considering that PerC B cells are primarily B1 cells (IgMhi, CD45R/B220lo, CD11bint) with a lesser but still significant fraction of B2 cells (IgM+, CD45R/B220hi, CD11b− Fig 2A), it was important to assess differences in the proliferative responses of these subsets. CFSE labeling revealed that COX inhibition significantly enhanced the B2 cell response (3.6% vs 17.8%) to BCR ligation whereas the response of B1 cells was limited (1.2% vs 2.0%; Fig. 7). IL-10 neutralization had no effect on the response to BCR ligation for either subset. B-1 cells had a small response to LPS (1.6 vs 3.0%) whereas B2 cells did not respond. IL-10 neutralization increased the LPS response of B2 cells (4.1 vs 14.6%) more than B1 cells (3.0 vs 6.7%). Interestingly, CD40 ligation led to the greatest division of both B cell subsets. These results illustrate that COX and IL-10 neutralization permit PerC B2 cell activation and that both subsets respond optimally to CD40 ligation.
Figure 7. CFSE reveals responses of B cell subsets.
CFSE-FITC-labeled PerC cells, were cultured with αIgM, LPS, or αCD40 +/− indomethacin or αIL-10, were resolved into B1 (CD45Rlo, CD11bint) and B2 (CD45Rhi, CD11b−) subsets by FACS analysis.
3.4 Cytokine impact upon the PerC B cell CD40 response
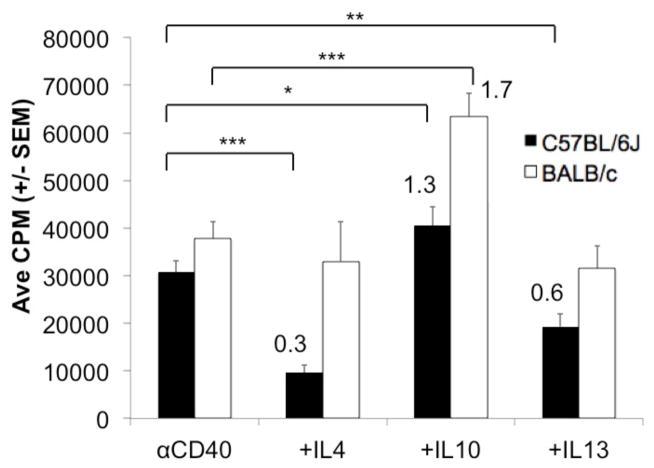
Considering the robust PerC B cell response to CD40 ligation, we tested if this response was susceptible to cytokine regulation as found with the BCR and LPS responses. While both IL-4 and IL-13 suppressed the C57BL/6J response to CD40, neither cytokine impacted the BALB/c response (Fig. 8). Considering that IL10 had no impact upon BCR ligation and suppressed the LPS response for both strains (Figs 4C, 5) it was interesting to see the CD40 response enhanced by exogenous IL-10, again for both strains. The homogeneity of BALB/c and C57BL/6J B cell responses to exogenous IL-10 contrasts that of IL-4 and IL-13 which differentially impact B cell activation based both on cell type and activation source.
Figure 8. Impact of cytokines on the PerC B cell proliferative response to CD40 ligation.
C57BL6/J or BALB/c PerC cells were activated with αCD40 MAb +/− exogenous IL-4, IL-10, or IL-13.
3.5 CD40 helps the suppressed BCR response but not that of LPS
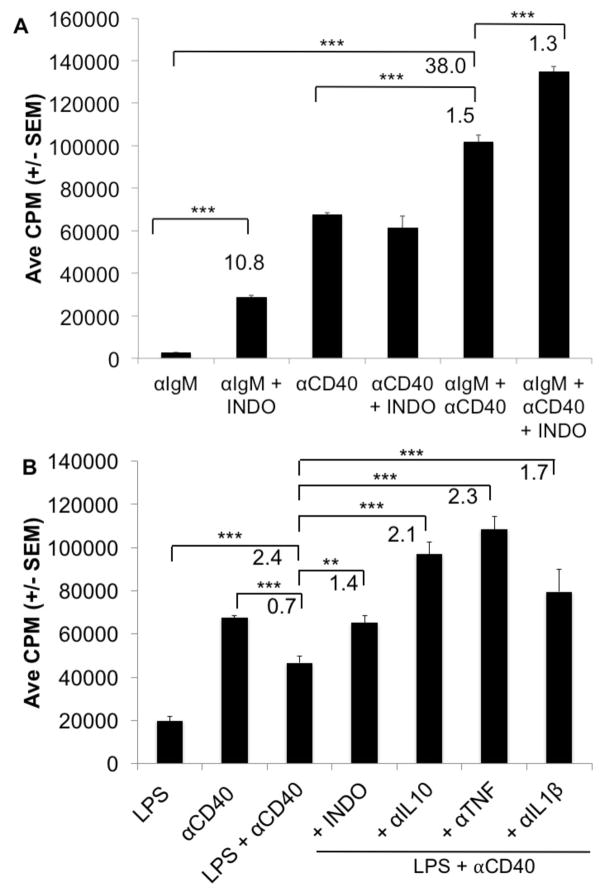
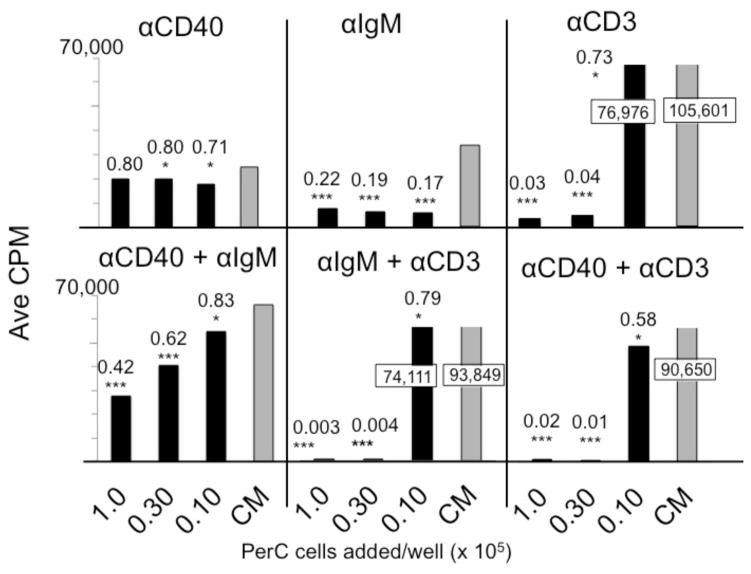
Since CD40 serves as a costimulatory molecule for B cells responding to thymus –dependent antigens it was logical to test if this signaling pathway could aid the suppressed PerC B cell response to BCR ligation [21]. Although the BCR response was markedly improved by CD40 costimulation (38-fold) there was still prostaglandin regulation as COX inhibition increased this response (Fig. 9A). In contrast, the CD40 response was suppressed by concurrent TLR-4 ligation and IL-10 neutralization permitted B cell activation (Fig 9B). Concurrent CD40 and TLR-4 ligation revealed regulation by TNF-α and IL-1β, cytokines with limited impact upon the PerC B cell response to either LPS or CD40 alone (not shown).
Figure 9. PerC B cell responses to BCR or TLR-4 ligation are enhanced by CD40 costimulation.
C57BL6/J PerC cells were activated with αIgM, αCD40, or αIgM + αCD40 +/− INDO (A). PerC cells were activated with LPS, αCD40, LPS + αCD40 +/− αIL10, αTNF, or αIL1β (B).
3.6 Macrophage regulation of T cells also suppresses CD40 activation of B cells
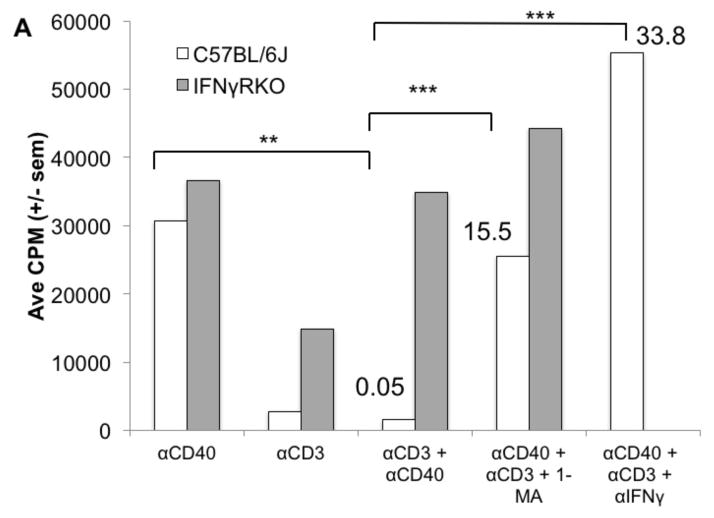
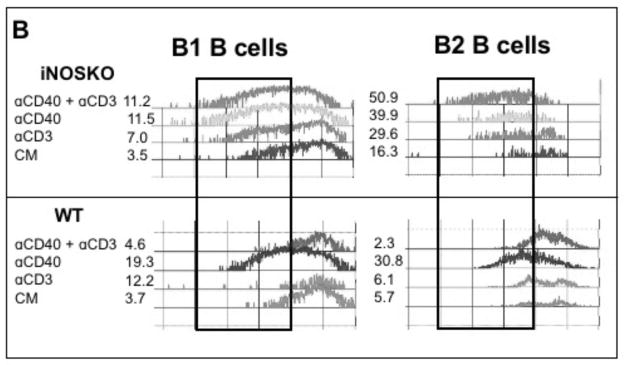
CD40 ligation was the only form of B cell activation that led to proliferation in the absence of exogenous enzyme inhibitors or cytokine-neutralizing MAbs. We questioned whether combining this form of stimulation, which enhances the APC function of both B cells and macrophages, with T cell activation could promote the PerC T cell proliferation normally restrained by macrophages. However, the suppression induced by TCR ligation abrogated the CD40 response (Fig. 10A). Genetic (IFNγR−/−) ablation and pharmacologic treatment (neutralizing anti-IFNγ MAb or iNOS inhibition by 1-MA) released the suppressed CD40 response (Fig 10A). CFSE analysis revealed that the CD40 response of both PerC B cell subsets was inhibited with CD3 ligation (Fig. 10B). However, neither subset was inhibited with cells from iNOS−/− mice during combined T and B cell activation. Thus, the IFNγ-triggered and iNOS-driven suppression generated during PerC T cell activation can prevent B cells from responding to CD40 ligation.
Figure 10. PerC T cell suppression overrides CD40 B cell activation.
C57BL/6J and IFNγRKO PerC cells were cultured with αCD40, αCD3, αCD40 + αCD3 +/− 1-MA or αIFNγ (A). C57BL/6J and iNOSKO PerC cells were labeled with CFSE-FITC and cultured with αCD3, αCD40, or αCD3 + αCD40 and cells were resolved into B1 and B2 B cell subsets by FACs analysis (B).
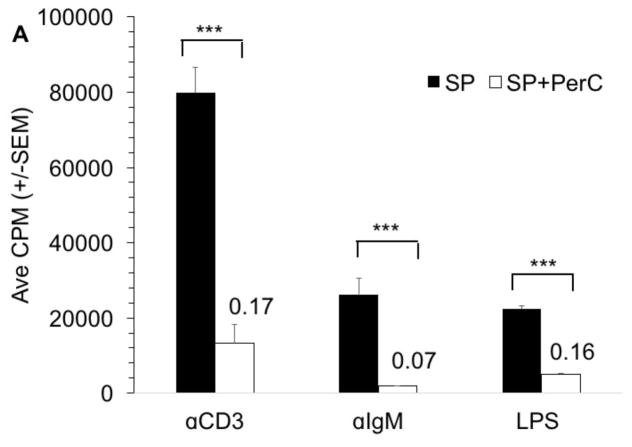
3.7 B cell costimulation can resist macrophage suppression
The normal (homeostatic) immune regulation found within the spleen or lymph node is distinct from that found within TMEs. Macrophages, compartmentalized within distinct regions of lymphoid organs, help initiate adaptive immunity whereas tumors with a disorganized and greater macrophage composition curb lymphocyte activation [4]. By titering macrophage-rich PerC cells into SP cell cultures we assessed which forms of lymphocyte activation were most susceptible or resistant to suppression. Small numbers (3.6 x 103) of macrophages reduced the SP T cell response by 27%; a 3-fold higher concentration of these cells essentially eliminated the response (≤ 4 % of control response) (Fig. 11). SP B cells responding to BCR ligation were sensitive to regulation losing 80% of the control response upon addition of the same, small number of macrophages. In contrast, regardless of macrophage concentration, the CD40 response was strong and much higher (70 – 80% of the control) than the response triggered by BCR ligation. With concurrent BCR and CD40 stimulation, all responses, based on CPM, were greater than either form of stimulation alone, but the response was still suppressed relative to the control and dropped with increasing macrophage number.
Figure 11. B cell co-stimulation and macrophage suppression in PerC plus SP cell co-cultures.
Spleen cells were cultured at 2 x 105 cells/well; titered PerC cells were added as indicated. CM control depicts SP cells cultured alone. Values in boxes indicate results that exceed the scale.
The CD40 response was suppressed in PerC cell cultures when the TCR was concurrently ligated (Fig. 10A). Macrophage titration progressively suppressed the spleen B cell response to CD40 when T cells were also activated (Fig. 11). These results reinforce that, although B cells respond to CD40 ligation in the presence of immunoregulatory macrophages, concurrent T cell activation overrides this response.
4. Discussion
The results reported here demonstrate that macrophages have the capacity to regulate B cell proliferation via pathways that differ based on the form of activation. While the response to ligation of the BCR was tempered by cyclooxygenase, IL-10 restrained the B cell response to LPS/TLR-4L. In contrast, macrophages had less impact on the CD40 response, which could reduce the suppression seen with simultaneous BCR or TLR-4 ligation. However, concurrent TCR ligation overrode the CD40 response. These results illustrate a hierarchy of macrophage control of lymphocyte activation under conditions that mimic the high myeloid-to-lymphoid ratio found within tumors.
In organized lymphoid tissue, T cells expressing CD154/CD40L ligate CD40 to promote macrophage, dendritic cell, and B cell maturation as evidenced by increased B7 and Class II MHC expression, production of pro- inflammatory (IL-6, IL-12, and TNFα) and counter-regulatory (IL-10) cytokines, and release of anti-microbial ROS and NO [22]. CD40-activated B cells secrete BAFF and APRIL, cytokines that help avert apoptosis of immature B cells [22]. In tumor tissue with a T cell infiltrate, CD40-stimulated macrophages create an environment of “simmering” inflammation that promotes angiogenesis, modifies tumor stroma, and tempers T cell activation [23,24]. As recently shown for pancreatic tumors, which tend to lack significant T cell infiltration, CD40 stimulation and chemotherapy can optimize myeloid activation and promote immunity, antagonizing the ability of IL10 to temper CTL production [25,26]. Thus, the imbalance of myeloid to lymphoid cells and degree of T cell activation (CD154 expression) in the TME can dictate the cytokine milieu and degree of activation or suppression therein. B cells within TMEs likely further contribute to this imbalance, responding to CD40 ligation with more IL-10 production [16–18].
LPS also activates B cells, dendritic cells, and macrophages but unlike CD40 ligation did not lead to significant PerC B cell proliferation unless IL-10 was neutralized. LPS triggers macrophages to produce ROS via NADPH oxidase (Nox4) [27]. Direct interaction between TLR-4 and Nox4 is essential for ROS and IL-10 production [28]. IL-10 reduces monocyte and macrophage transcription of IL-1β, IL-6, and TNF-α, the key pro-inflammatory cytokines produced by cells that respond to LPS [29]. TLR-activated B cells, via IL-10 production, promote self-tolerance in TH1 and TH17 cells [30]. BCR ligation also triggers ROS production, which, although essential for full activation, tempers B cell proliferation [31]. ROS triggers COX in macrophages to produce PGE2, which has direct effects on B cell proliferation and antibody production and promotes TH2 polarization [32–36]. The growth suppressive effect of PGE2 is mediated via binding EP4, a PGE2R and candidate tumor suppressor [37]. CD40-activated B cells resist PGE2 and have been suggested to serve as APCs for tumor vaccination within the suppressive TME [38–40]. Regardless of the form of B cell stimulation, regulatory mechanisms, mediated by macrophages and dendritic cells, are initiated upon activation (41,42). These anti-inflammatory constraints on immunity can be co-opted to contribute to the development of the ROS- and IL-10-rich, immunosuppressive TME [43–45].
PerC B cell subsets differed in their release from suppression with B2 cells having better responses to both BCR and TLR-4 ligation. B1 cells are known to differ from B2 cells in their failure to proliferate in response to BCR ligation, which is postulated to reflect increased phosphatase activity and IL10 production [15, 46–49]. Unlike B2 cells, B1 cells had a measurable LPS response prior to IL10 neutralization. However, the response of B2 cells was greater with IL10 blockade. Both B cell subsets responded best to CD40 ligation. Activation of B1 cells promotes their production of autocrine IL-10, a property shared with the B10/Breg cells that are best driven to IL-10 production by CD40 ligation [16]. B10/Breg cells suppress pathogenic TH1 and TH17 cells and induce production of Tregs [50]. Although B2 cells generally responded better than B1 cells under macrophage-dense conditions, this environment has the potential to promote B2 cell conversion into B1 cells, generating cells with enhanced survival capability [51, 52]. Considering the potent ability of IL-10 to temper cell-mediated immunity, this characteristic is perhaps most problematic for B cell activation in TMEs.
Putative ligands for activating B cells within macrophage-rich TMEs are numerous. B1 cells have polyspecific or “natural antibody” receptors that might bind N-glycosylated gangliosides, oxidized lipids, phosphatidylcholine, cancer-derived microvesicles or exosomes, and apoptotic debris, all antigens likely enriched within TMEs [53–55]. Furthermore, the TLR-4 ligands HMGB-1, HSP, hyaluronan, and heparin sulfate are self-antigens found in TMEs [56]. Trafficking with macrophages, B1 cells have been implicated in anti-inflammatory tissue “housekeeping” and apoptotic corpse clearance [15]. Furthermore, several forms of cancer have been found to enhance B1 B cell survival, increasing IL-10 production and suppressing CTL effector generation [57–60]. Thus, TMEs likely provide a milieu that can activate B1 and B10/Breg cells or drive B2 cells to become B10/Breg cells. Such cells can facilitate generation of Tregs , polarize macrophages and foster the maturation of myeloid-derived suppressor cells (MDSCs), potent inhibitors of cell-mediated immunity [17,18,61–63].
PerC cell culture can serve as an in vitro system to model the multifaceted, aberrant immune regulation found within macrophage-rich TMEs. The complexity found therein is exemplified by the difference in lymphocyte costimulatory responses found for B versus T lymphocytes, where B cell costimulation through CD40 reduced and T cell costimulation via CD28 increased, suppression [14]. That suppression manifest by T cell activation was dominant to the CD40 response is further evidence of the challenge in promoting immunity under macrophage-dense conditions. Deciphering the contribution of genetics (BALB/c, C57BL/6, etc.), immune checkpoint receptor/ligand combinations (PD/PDL1/2, CTLA4/CD80/6, etc.), sterile inflammation receptors (CD36, TLRs, etc.), and the cytokine (TH1, TH2, TH17, etc.) milieu to the impaired immunity generated by macrophage-dense conditions becomes pliable with the mouse PerC cell culture model. By advancing knowledge of which conditions best promote immunity these studies could inform strategies for restoring immunity in TMEs and optimizing cancer vaccine design [64].
Highlights.
Peritoneal cell culture models macrophage-rich tumor microenvironments (TMEs)
Cyclooxygenase and IL10 suppress B cells activated by BCR and TLR-4 ligation
Cultured peritoneal cavity B cells respond to CD40 ligation
CD40 ligation enhanced BCR or TLR-4 responses but was suppressed by concurrent TCR ligation
Acknowledgments
This research was supported by the NIH AREA program (R15 AI 060356-01, R15 CA 136901-01). A. Swider was supported by a Rider University Undergraduate Research Scholar Award. We are grateful to Gabriella Chaviano, Jackelyn Cua, Brian Deeney, Ashley O’Brien, Steve Sacavage, and Amy Werda for help with early experiments and E. Lomakova, D. Pastuna, and G. Torres for mouse husbandry.
Abbreviations
- APC
antigen presenting cell
- ARG
arginase
- COX
cyclooxygenase
- IDO
indoleamine 2,3-dioxygenase
- INDO
indomethacin
- iNOS
inducible nitric oxide synthase
- PerC
peritoneal cavity
- ROS
reactive oxygen species
- SP
spleen
- 1-MA
NG-monomethyl-L-arginine
- 1-MT
1 methyl tryptophan
- 1-NA
N-w-hydroxy-nor-L-arginine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kelly P, Davison R, Bliss E, McGee J. Macrophages in human breast disease: a quantitative immunohistochemical study. Br J Cancer. 1988;57:174–177. doi: 10.1038/bjc.1988.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaput N, Svrcek M, Auperin A, Locher C, Drusch F, Malka D, Taieb J, Goere D, Ducreux M, Boige V. Tumour-infiltration CD68+ and CD57+ cells predict patient outcome in stage II–III colorectal cancer. Br J Cancer. 2013;109:1013–1022. doi: 10.1038/bjc.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo Z, Wang Q, Lau WB, Lau B, Xu L, Zhao L, Yang H, Feng M, Xuan Y, Yang Y, Lei L, Wang C, Yi T, Zhao X, Wei Y, Zhou S. Tumor microenvironment: the culprit for ovarian cancer metastasis? Cancer Lett. 2016;377:174–182. doi: 10.1016/j.canlet.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 4.Wynn T, Chawla A, Pollard J. Macrophage biology in development, homeostasis, and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahoney K, Rennert P, Freeman G. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–584. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 6.West N, McCuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15:615–629. doi: 10.1038/nri3896. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Flies D. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smyth M, Ngiow S, Ribas A, Teng M. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13:143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30:668–681. doi: 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz M, Zhang Y, Rosenblatt J. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer. 2016;194:40. doi: 10.1186/s40425-016-0145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matlack R, Yeh K, Rosini L, Gonzalez D, Taylor J, Silberman D, Pennello A, Riggs J. Peritoneal macrophages suppress T-cell activation by amino acid catabolism. Immunology. 2006;117:386–395. doi: 10.1111/j.1365-2567.2005.02312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Composto G, Gonzalez D, Bucknum A, Silberman D, Taylor J, Kozlowski M, Bloomfield T, Bartlett T, Riggs J. Peritoneal T lymphocyte regulation by macrophages. Immunobiology. 2011;216:256–264. doi: 10.1016/j.imbio.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silberman D, Bucknum A, Kozlowski M, Matlack R, Riggs J. Cytokine treatment of macrophage suppression of T cell activation. Immunobiology. 2010;215:70–80. doi: 10.1016/j.imbio.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silberman D, Bucknum A, Bartlett T, Composto G, Kozlowski M, Walker A, Werda A, Cua J, Sharpe A, Somerville J, Riggs J. CD28 ligation increases macrophage suppression of T-cell proliferation. Cell Mol Immunol. 2012;9:341–349. doi: 10.1038/cmi.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 16.Tedder T. B10 cells: a functionally defined regulatory B cell subset. J Immunol. 2015;194:1395–1401. doi: 10.4049/jimmunol.1401329. [DOI] [PubMed] [Google Scholar]
- 17.Olkhanud P, Damdinsuren B, Bodogai M, Gress R, Sen R, Wejksza K, Malchinkhuu E, Wersto R, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei X, Jin Y, Tian Y, Zhang H, Wu J, Lu W, Lu X. Regulatory B cells contribute to the impaired antitumor immunity in ovarian cancer patients. Tumor Biol. 2015 doi: 10.1007/s13277-015-4538-0. doi:10.1007. [DOI] [PubMed] [Google Scholar]
- 19.Ren Z, Peng H, Fu Y. PD-1 shapes B cells as evildoers in the tumor microenvironment. Cancer Discovery. 2016;6:477–478. doi: 10.1158/2159-8290.CD-16-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunderson A, Kaneda M, Tsujikawa T, Nguyen A, Affara N, Ruffell B, Gorjestani S, Liudahl S, Truitt M, Olson P, Kim G, Hanahan D, Tempero M, Shepard B, Irving B, Chang B, Varner J, Coussens L. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 2016;6:270–285. doi: 10.1158/2159-8290.CD-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parry S, Hasbold J, Holman M, Klaus G. Hypercross-linking surface IgM or IgD receptors on mature B cells induces apoptosis that is reversed by costimulation with Il-4 and anti-CD40. J Immunol. 1994;152:2821–2829. [PubMed] [Google Scholar]
- 22.Daphne Y, Clark E. The role of CD40 and CD40L in dendritic cells. Semin Immunol. 2005;21:265–272. doi: 10.1016/j.smim.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rammello M, Tossello Boari J, Canale F, Mena H, Negrotto S, Gastman B, Gruppi A, Acosta Rodriguez E, Montes C. Tumor-induced senescent T cells promote the secretion of pro-inflammatory cytokines and angiogenic factors by human monocytes/macrophages through a mechanism that involves Tim-3 and CD40L. Cell Death Dis. 2014 Nov 6;5:e1507. doi: 10.1038/cddis.2014.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beatty G, Chiorean E, Fishman M, Saboury B, Teitelbaum U, Sun W, Huhn R, Song W, Li D, Sharp L, Torigian D, O’Dwyer P, Vonderheide R. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrne K, Vonderheide R. CD40 stimulation obviates innate sensors and drives T cell immunity in cancer. Cell Report. 2016;15:2719–2732. doi: 10.1016/j.celrep.2016.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brossart P, Zobywalski A, Grunebach F, Behnke L, Stuhler G, Reichardt V, Kanz L, Brugger W. Tumor necrosis factor alpha and CD40 ligand antagonize the inhibitory effects of IL10 on T-cell stimulatory capacity of dendritic cells. Cancer Res. 2000;15:4485–4492. [PubMed] [Google Scholar]
- 27.Sumimoto H, Miyano K, Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- 28.Deng J, Wang X, Qian F, Vogel S, Xiao L, Ranjan R, Park H, Karpurapu M, Ye R, Park G, Christman J. Protective role of reactive oxygen species in endotoxin-induced lung inflammation through modulation of IL-10 expression. J Immunol. 2012;188:5734–5740. doi: 10.4049/jimmunol.1101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen HH, Tran BT, Muller W, Jack R. IL-10 acts as a developmental switch guiding monocyte differentiation to macrophages during a murine peritoneal infection. J Immunol. 2012;189:3112–3120. doi: 10.4049/jimmunol.1200360. [DOI] [PubMed] [Google Scholar]
- 30.Lamropoulou V, Hoehlig K, Roch T, Neves P, Calderon Gomez E, Sweenie C, Hao Y, Freitas A, Steinhoff U, Anderton S, Fillatreau S. TLR-activated B cells suppess T cell-mediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 31.Richards S, Clark E. BCR-induced superoxide negatively regulates B-cell proliferation and T-cell-independent type 2 Ab responses. Eur J Immunol. 2009;39:3395–3403. doi: 10.1002/eji.200939587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurland J, Kincaid P, Moore M. Regulation of B-lymphocyte clonal proliferation by stimulatory and inhibitory macrophage-derived factors. J Exp Med. 1977;146:1420–1435. doi: 10.1084/jem.146.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheeler M, DeFranco A. Prolonged production of reactive oxygen species in response to B cell receptor stimulation promotes B cell activation and proliferation. J Immunol. 2012;189:4405–4416. doi: 10.4049/jimmunol.1201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chace J, Fleming A, Gordon J, Perandones C, Cowdery J. Regulation of differentiation of peritoneal B-1a (CD5+) B cells. Activated peritoneal macrophages release prostaglandin E2, which inhibits IgM secretion by peritoneal B-1a cells. J Immunol. 1995;154:5630–5636. [PubMed] [Google Scholar]
- 35.Simkin N, Jelinek D, Lipsky P. Inhibition of B cell responsiveness by prostaglandin E2. J Immunol. 1987;138:1074–1081. [PubMed] [Google Scholar]
- 36.Fedyk E, Phipps R. Prostaglandin E2 receptors of the EP2 and EP4 subtypes regulate activation and differentiation of mouse B lymphocytes to IgE-secreting cells. Proc Natl Acad Sci. 1996;93:10978–10983. doi: 10.1073/pnas.93.20.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murn J, Alibert O, Wu N, Tendil S, Gidrol X. Prostaglandin E2 regulates B cell proliferation through a candidate tumor suppressor, Ptger4. J Exp Med. 2008;205:3091–3103. doi: 10.1084/jem.20081163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcelletti J. IL-10 inhibits lipopolysaccharide-induced murine B cell proliferation and cross-linking of surface antigen receptors or ligation of CD40 restores the response. J Immunol. 1996;157:3323–3333. [PubMed] [Google Scholar]
- 39.Shimabukuro-Vornhagen A, Draube A, Liebig T, Popov A, Rothe A, von Bergwelt-Baildon M. The properties of human CD40-activated B cells as antigen presenting cells are not affected by PGE2. Oncol Rep. 2013;29:1061–1065. doi: 10.3892/or.2012.2215. [DOI] [PubMed] [Google Scholar]
- 40.Magari M, Nishikawa Y, Fujii Y, Nishio Y, Watanabe K, Fujiwara M, Kanayam N, Ohmori H. IL-21 dependent B cell death driven by prostaglandin E2, a product secreted from follicular dendritic cells. J Immunol. 2011;187:4210–4218. doi: 10.4049/jimmunol.1100934. [DOI] [PubMed] [Google Scholar]
- 41.Santos L, Draves K, Boton M, Grewal P, Marth J, Clark E. Dendritic cell-dependent inhibition of B cell proliferation requires CD22. J Immunol. 2008;180:4561–4569. doi: 10.4049/jimmunol.180.7.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sindhava V, Tuna H, Gachuki BW, DiLillo D, Avdiushko M, Onami T, Tedder T, Cohen D, Bondada S. Bone marrow dendritic cell-mediated regulation of TLR and B cell receptor signaling in B cells. J Immunol. 2012;189:3355–3367. doi: 10.4049/jimmunol.1101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y, Antony S, Meitzler J, Doroshow J. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014;335:164–173. doi: 10.1016/j.canlet.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans R. Regulation of T- and B lymphocyte responses to mitogens by tumor-associated macrophages: the dependency on the stage of tumor growth. J Leuk Biol. 1984;35:549–559. doi: 10.1002/jlb.35.6.549. [DOI] [PubMed] [Google Scholar]
- 45.Lu T, Gabrilovich D. Molecular pathways: tumor-infiltrating myeloid cells and reactive oxygen species in the regulation of tumor microenvironment. Clin Cancer Res. 2012;18:4877–4882. doi: 10.1158/1078-0432.CCR-11-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayakawa K, Hardy R, Parks D, Herzenberg L. The “Ly-1 B” subpopulation in normal, immunodefective, and autoimmune mice. J Exp Med. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayakawa K, Hardy R, Honda M, Herzenberg L, Steinberg A, Herzenberg L. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci. 1984;81:2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holodick N, Rothstein T. Atypical response of B-1 cells to BCR ligation: a speculative model. Front Immunol. 2013;4:457. doi: 10.3389/fimmu.2013.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alhakeem S, Sindhava V, McKenna M, Gachuki B, Byrd J, Muthusamy N, Bondada S. Role of B cell receptor signaling in IL-10 production by normal and malignant B-1 cells. Ann NY Acad Sci. 2015;1362:239–249. doi: 10.1111/nyas.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mauri C, Bosma A. Immune Regulatory Function of B cells. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 51.Wortis H, Teutsch M, Higer M, Zheng J, Parker D. B-cell activation by crosslinking of surface IgM or ligation of CD40 involves alternative signal pathways and results in different B-cell phenotypes. Proc Natl Acad Sci. 1995;92:3348–3352. doi: 10.1073/pnas.92.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thies F, Lucatelli Fernanda Lucatelli Laurindo M, Perez E, Romulo Novaes e Brito R, Mariano M, Flavia Popi A. Cross talk between peritoneal macrophages and B-1 cells in vitro. PLoS One. 2013;8:e62805. doi: 10.1371/journal.pone.0062805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez-Zhurbenko N, Rabade-Chediak M, Martinez D, Grinan T, Hernandez AM. Anti-NeuGcGM3 reactivity: a possible role of natural antibodies and B-1 cells in tumor immunosurveillance. Ann NY Acad Sci. 2015;1362:224–238. doi: 10.1111/nyas.12827. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, An J, Huang S, He J, Zhang J. Esophageal cancer-derived microvesicles induce regulatory B cells. Cell Biochem Funct. 2015;33:308–313. doi: 10.1002/cbf.3115. [DOI] [PubMed] [Google Scholar]
- 55.Binder C, Papac-Milicevic N, Witzum J. Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol. 2016;16:485–497. doi: 10.1038/nri.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sato Y, Goto Y, Narita N, Hoon D. Cancer cells expressing toll-like receptors and the tumor microenvironment. Cancer Microenviron. 2009;1:205–214. doi: 10.1007/s12307-009-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inoue S, Leitner W, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 58.Shao Y, Lo C, Ling C, Liu X, Ng K, Chu A, Ma Y, Li C, Fan S, Man K. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett. 2014;355:264–272. doi: 10.1016/j.canlet.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 59.Laurindo M, Thies F, Perez E, Novaes e Brito R, Mariano M, Popi A. B16 melanoma cells increase B-1 cell survival, IL-10 production and radioresistance in vitro. Immunobiol. 2013;218:609–619. doi: 10.1016/j.imbio.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 60.Lee K, Spata M, Bayne L, Buza E, Durham A, Allman D, Vonderheide R, Simon M. Hif1a deletion reveals pro-neoplastic function of B cells in pancreatic cancer. Cancer Discov. 2016;6:256–269. doi: 10.1158/2159-8290.CD-15-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bodogai M, Moritoh K, Lee-Chang C, Hollander C, Sherman-Baust C, Wersto R, Araki Y, Miyoshi I, Yang L, Trinchieri G, Biragyn A. Immunosuppressive and prometastatic functions of myeloid-derived suppressive cells rely upon education from tumor associated B cells. Cancer Res. 2015;75:3456–3465. doi: 10.1158/0008-5472.CAN-14-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X, Abastado JP, Lam KP, Biswa S. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol. 2010;40:2296–2307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 63.Margry B, Kersemakers S, Hoek A, Arkestein G, Wieland W, van Eden W, Broere F. Activated peritoneal cavity B-1a cells possess regulatory B cell properties. PLoS One. 2014;9:e88869. doi: 10.1371/journal.pone.0088869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DiLillo D, Yanaba K, Tedder T. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth. J Immunol. 2010;184:4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]