Fig. 5. Unique insights from the in silico molecular docking studies of EGCG binding on IRAK-1, TAK1-TAB1 complex, and TRAF6. (A).
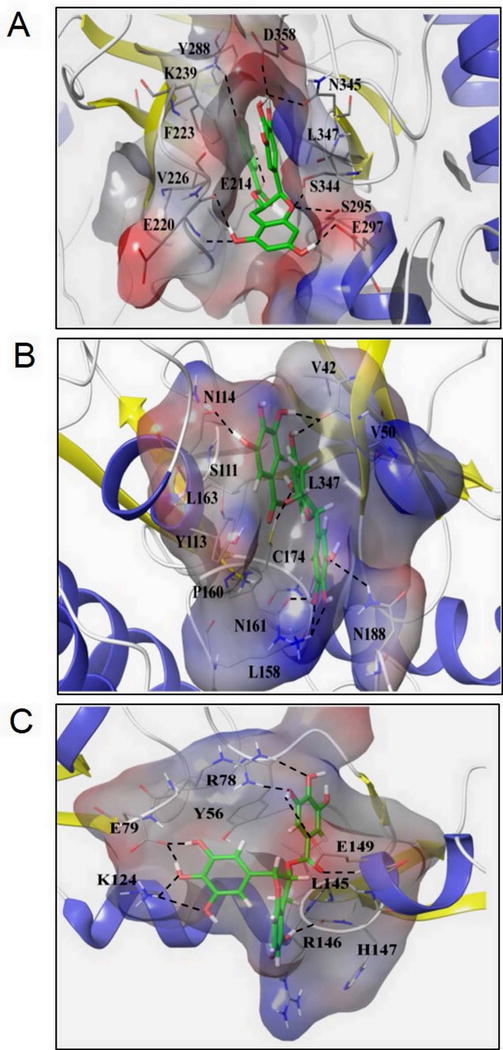
Molecular docking of EGCG on a constructed IRAK-1 structure showed the hydroxyl group of the trihydroxyphenyl ring of EGCG perfectly positions (1.6 Å) to make an H-bond with D358, a conserved aspartate residue and ATP-binding site, and N345, which inhibits IRAK-1 kinase activity. (B) EGCG forms an H-bond at C174, an ATP-binding site, on the TAK1-TAB1 complex and stabilizes its position in the binding pockets by forming hydrophobic links to distal V42, V50, and L163 residues that happen to be TAK1 nucleotide binding regions and a π-π interaction at Y113, thus serving as a potent and reversible TAK1 inhibitor. (C) Side chains of E79 and K124 residues on TRAF6 play significant roles in fastening the position of trihydroxyphenyl ring of EGCG through the H-bond network, thereby inhibiting its K63 autoubiquitination.
