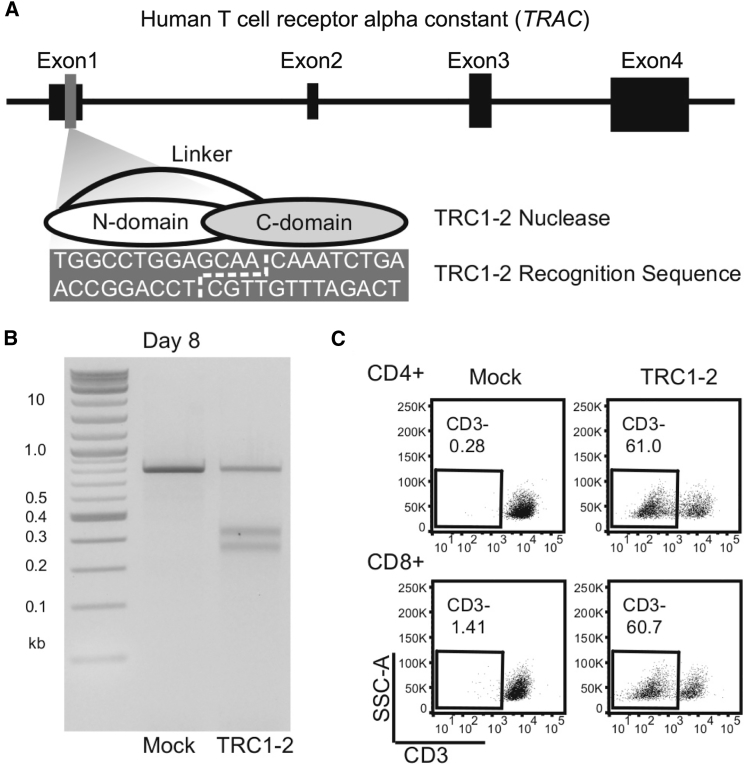
Figure 1.
Characterization of TRC1-2 Nuclease Activity in T Cells
(A) Diagram of the TRC1-2 nuclease and recognition site within the TRAC locus. The TRC1-2 nuclease is a single-chain protein consisting of an N-terminal domain (N-domain) and C-terminal domain (C-domain) connected by a flexible linker. The recognition site consists of 9-bp half-sites recognized by each of the two nuclease domains, separated by a 4-bp central sequence. A broken white line in the recognition sequence denotes the overhangs generated following cleavage by the TRC1-2 nuclease. (B) A T7 endonuclease (T7E) assay was performed on mock-electroporated T cells and T cells treated with TRC1-2 nuclease on day 8 post-electroporation to confirm editing at the TRAC locus. (C) Flow cytometry staining of CD3 expression in CD4+ and CD8+ T cells on day 8 post-electroporation with TRC1-2 nuclease. Reduction of cell surface expression of CD3, a component of the TCR complex, is a functional marker of disruption of TCRα expression.