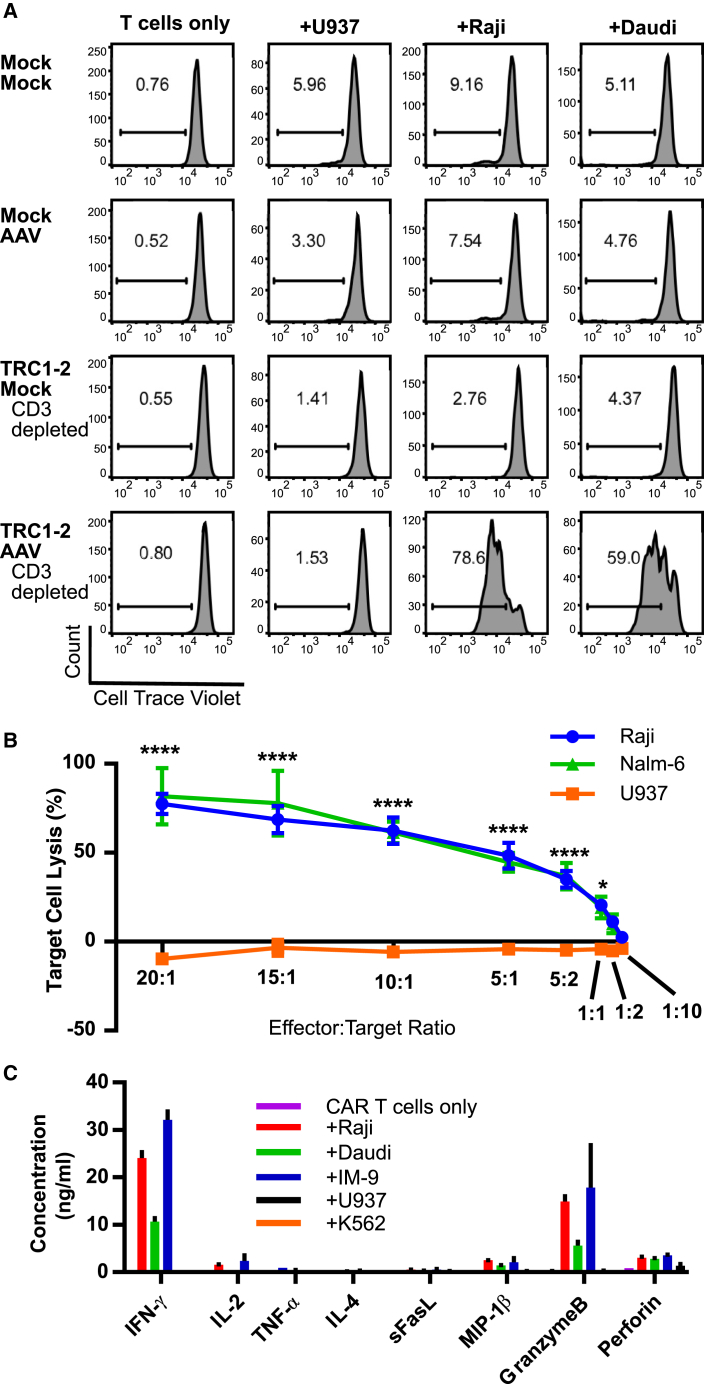
Figure 5.
In Vitro Activity of Gene-Edited TCR Knockout Anti-CD19 CAR+ Cells
(A) Cells were either mock-electroporated or electroporated with TRC1-2 mRNA (TRC1-2) and then immediately split into two groups; one was mock-transduced, and one was transduced with AAV:TRAC:CAR (AAV) at an MOI of 50,000 vg/cell. CD3+ cells were depleted from both TRC1-2 mRNA-treated groups post-electroporation or post-electroporation and transduction with AAV. T cells from all four groups were labeled with Cell Trace Violet and then cultured alone or co-cultured at a ratio of 1:1 with control CD19− U937 cells or CD19+ Raji or Daudi cells. All cell lines were pre-treated with Mitomycin C to arrest cell growth and washed extensively prior to co-culture. After 3 days of co-culture in medium in the absence of exogenous cytokines, proliferation (dilution of Cell Trace Violet) was assessed by flow cytometry. (B and C) TCR knockout CAR+ T cells were produced by electroporation of T cells with TRC1-2 mRNA, followed immediately by transduction with AAV:TRAC:CAR at an MOI of 400,000. Cells were depleted of CD3+ cells 5 days post-electroporation and transduction. (B) TCR knockout CAR+ T incubated with Raji (CD19+), NALM-6 (CD19+), or U937 (CD19−) cells at various effector:target ratios. Cytolytic activity of the CAR T cells against the Raji, NALM-6, or U937 targets was measured by assessment of LDH release. Data are from n = 7 individual wells per sample per time point, mean ± SEM, ****p < 0.0001, *p < 0.05, two-way ANOVA with Tukey multiple comparisons test comparing Raji or NALM-6 to U937 samples at the same E:T ratio. (C) CAR T cells were incubated alone or co-cultured at a ratio of 10:1 with control CD19− U937 or K562 cells or CD19+ Raji, Daudi, or IM-9 cells for 24 hr in medium in the absence of exogenous cytokines. Cytokine production and release were quantified from culture supernatants (n = 3, mean ± SEM).