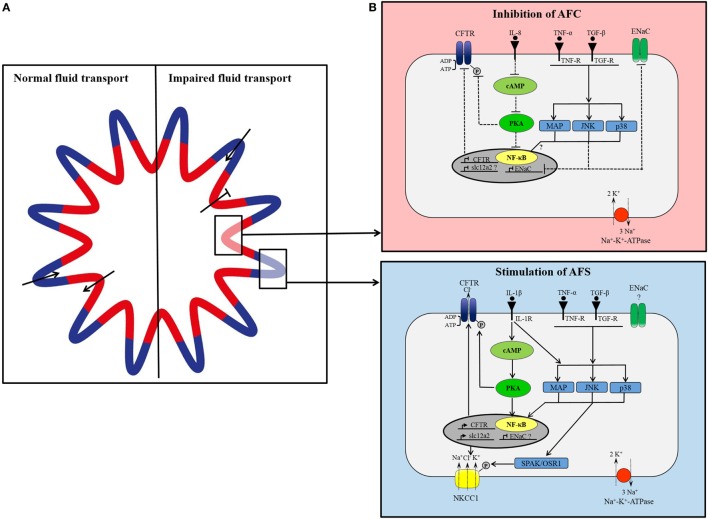
Figure 2.
(A) Proposed model of organization of small airways. In lung epithelium, independent groups of cells simultaneously secrete and absorb to maintain fluid homeostasis. Under normal conditions, cells located in the pleats secrete fluid (blue) and cells located around the folds concurrently absorb secreted fluid (red). In lung injury, fluid transport in absorptive areas may be blocked while fluid secretion stays intact or increases. (B) Simplified, hypothetical concept of differential cytokine-dependent regulation of ion transporter in absorptive (red) and secretory (blue) areas in inflammatory lung disease. In apsorptive areas, AFC is impaired presumably through cytokine-mediated inhibition of CFTR and epithelial Na+ channel (ENaC). Proinflammatory cytokines [tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), interleukin1β (IL-1β)] bind to their receptor inducing intracellular signaling cascades of MAP-kinases, JNK, p38, which prevent expression of ENaC. IL-8 blocks CFTR expression and activation by inhibition of cAMP/PKA pathway. In secretory areas, fluid secretion is stimulated by cytokines. Receptor binding of IL-1β, TNF-α, and TGF-β induces intracellular signaling pathways leading to activation and expression of CFTR and NKCC1.