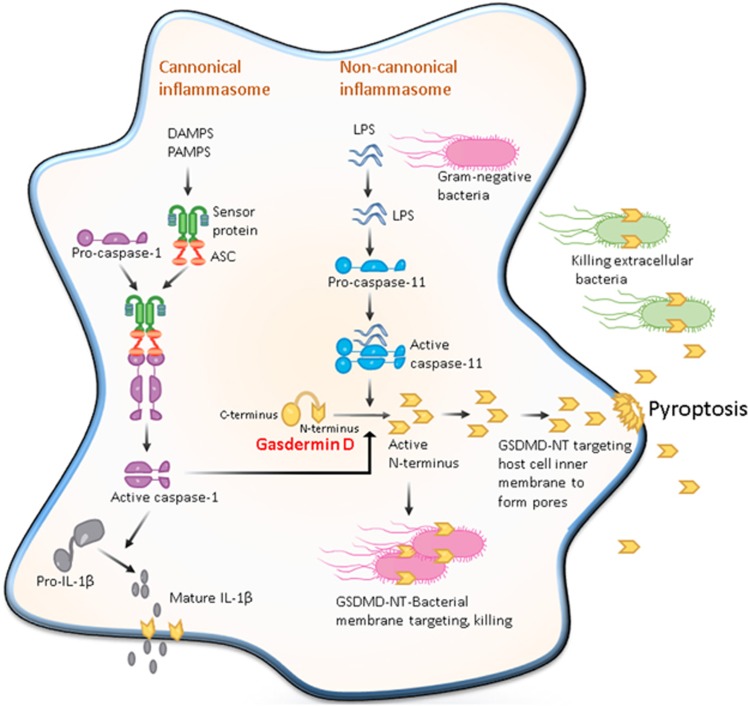
Figure 1.
Pore-forming activity of GSDMD determines pyroptotic cell death. The canonical inflammasome pathway is triggered by different cytoplasmic sensor proteins recognizing multiple pathogens and inflammatory agents. These recruit procaspase-1 monomers through the adaptor protein ASC and activate the caspase by dimerization. Caspase-1 can also initiate pyroptosis by cleaving gasdermin D although other pyroptosis-inducing caspase-1 substrates may exist. Caspase-1 also processes the proinflammatory cytokine pro-IL-1β to generate mature IL-1β, which is presumably released by cell lysis during pyroptosis. The non-canonical inflammasome pathway is triggered by bacterial lipopolysaccharide (LPS) molecules in the cytoplasm of infected cells. Direct binding of LPS to the protein procaspase-11 causes the protein dimerization to become active caspase-11. In human cells, caspase-4 and -5 perform the function of mouse caspase-11. These caspases cleave the protein gasdermin D, releasing its amino-terminal domain from inhibition by its carboxy-terminal domain. The cytotoxic N-terminal fragment of gasdermin D is then released and targets phospholipids on the host cell membrane and assembled into the pores. Processed GSDMD-NT, via its affinity for cardiolipin and phosphatidylserine, may also target and kill intracellular bacteria. GSDMD-NT pore formation in the host cell leads to pyroptosis to produce apoptotic body-like cell protrusions. IL-1β is released from the cell upon membrane rupture