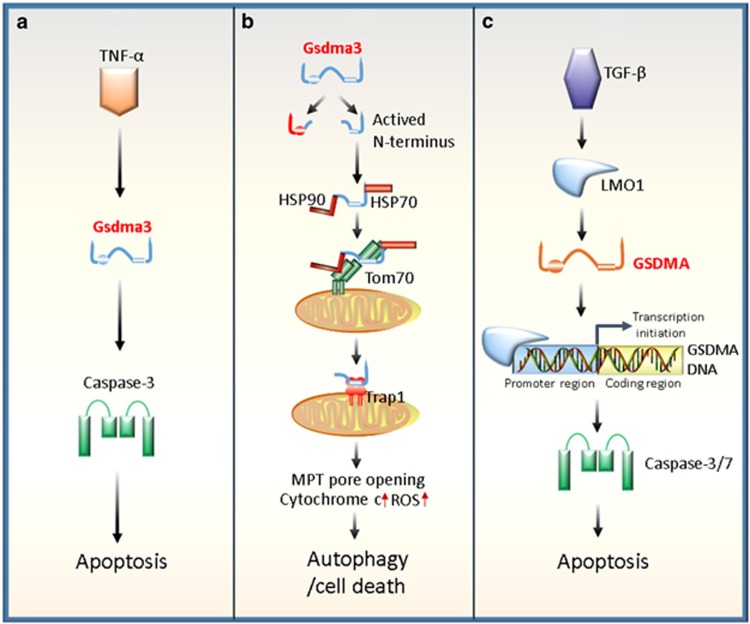
Figure 2.
Regulatory roles of Gsdma3/GSDMA in autophagy or apoptosis. (a) Gsdma3 acts as a key mediator in the TNF-α-induced apoptosis pathway. TNF-α can upregulate Gsdma3, whereas Gsdma3 causes the catagen-associated apoptosis of hair follicle keratinocytes by directly enhancing the caspase-3 expression. (b) Gsdma3 causes autophagy through a mitochondria-dependent pathway. Mutant Gsdma3 loses its whole C-terminal domain, consequently releasing the intrinsic pro-autophagic activity of the N-terminal domain. Then, the unmasked N-terminal domain of Gsdma3 is associated with Hsp90 to be delivered to mitochondria through mitochondrial importer receptor Tom70, where it interacts with the mitochondrial chaperone Trap1 and induces mitochondrial permeability transition (MPT) pore opening, mitochondrial reactive oxygen species (ROS) production, cytochrome c releasing, resulting finally in cell death. (c) Human GSDMA involves transforming growth factor (TGF)-β-induced apoptosis. TGF-β upregulates GSDMA expression by LIM domain only 1 (LMO1) induction through a sequence to which LMO1 binds, in a GSDMA promoter region. Ultimately, the increased GSDMA induces apoptosis of the pit cells of human gastric epithelium