Figure 1.
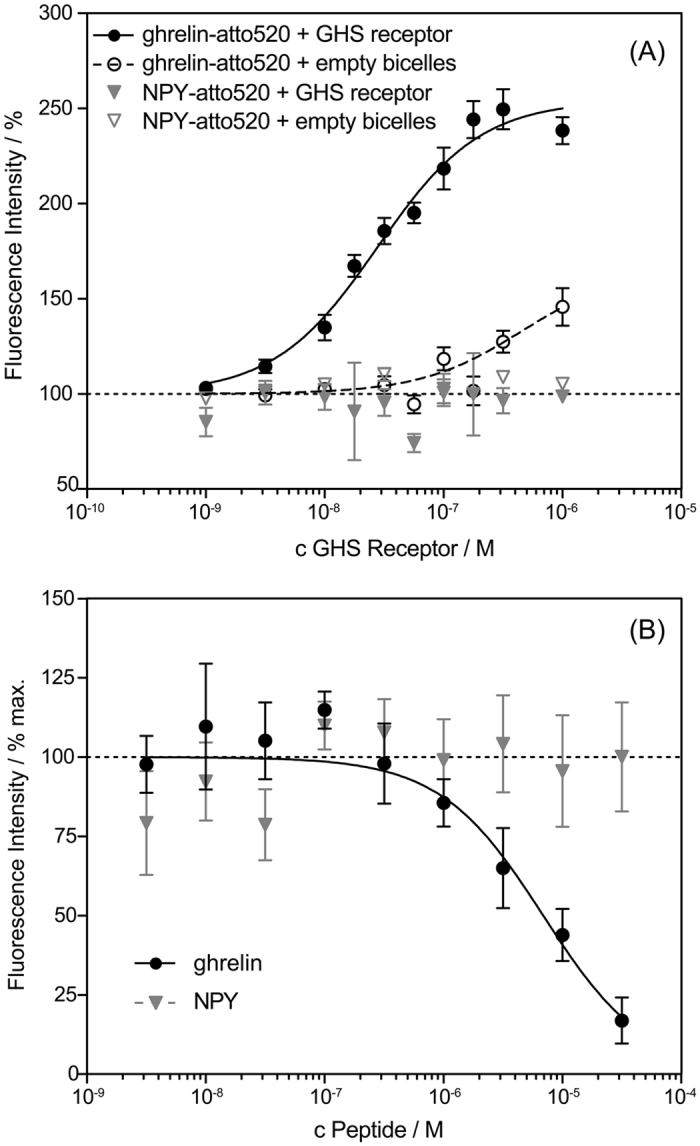
(A) Saturation binding of atto520-labeled ghrelin (c = 100 nM) to increasing amounts of GHS receptor-containing bicelles or empty bicelles. As a control, atto520-labeled NPY was used, which did not display enhanced fluorescence in the presence of GHS receptor-loaded or empty bicelles. Data reflect fluorescence enhancement upon binding. The inflection point (EC50 = 28 nM) for GHS receptor binding is approximately at the limit of the assay of Ltotal/2 = 50 nM; demonstrating high functionality of the system. (B) Displacement of atto520-ghrelin binding to the GHS receptor by unlabeled ghrelin. In contrast, neuropeptide Y (NPY) was not able to displace the bound atto520-ghrelin ligand. Results represent mean +/− SEM of three independent assays each performed in triplicates.
