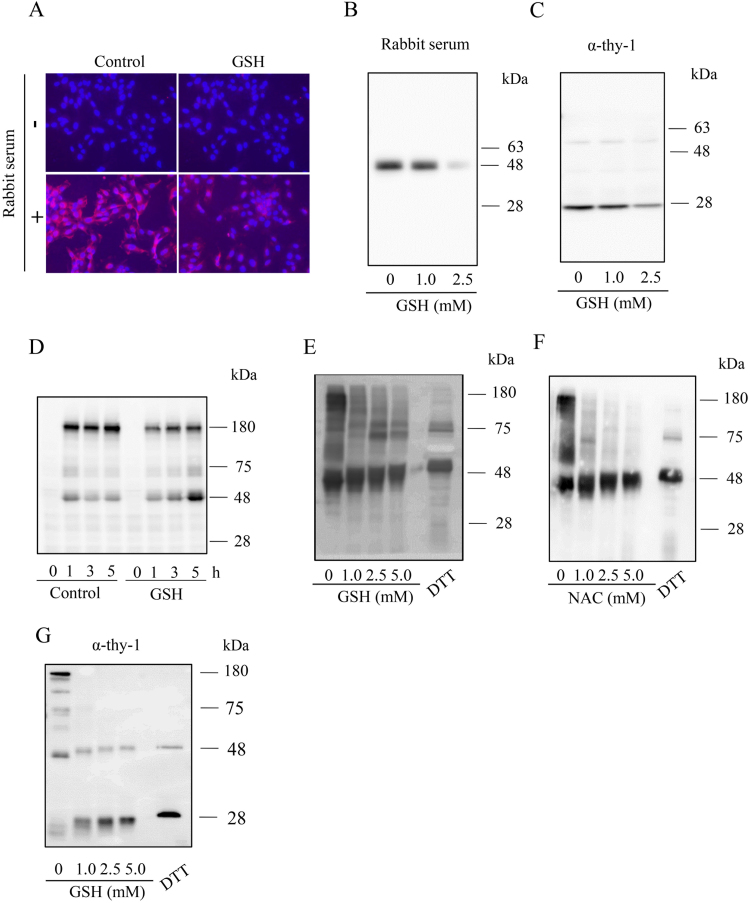
Fig. 3.
GSH inhibits the binding of rabbit and mouse Igs to MC surface and promotes the cleavage of the disulfide bonds in Igs. (A-D) Effect of GSH on antibody binding to the cell surface. (A) Immunofluorescence staining of membrane-bound IgG. MCs were incubated with 3% rabbit serum for 2 h in the presence or absence of 5 mM GSH. The presence of rabbit Igs in cell surface was detected with rhodamine-conjugated anti-rabbit IgG antibody. Note the high red fluorescence in MCs and its prevention by GSH. (B, C) Precipitation of the cell membrane-bound Igs using protein A/G beads. MCs were exposed to 1% heat-treated rabbit serum (B) or 1.0 μg/ml anti-Thy-1 antibody (C) in the presence or absence of the indicated concentration of GSH for 30 min. After washing out the unbound free Igs, cells were lysed with SDS buffer. The cell-bound Igs were precipitated and subjected to Western blot analysis using an HRP-labeled anti-rabbit or anti–mouse antibody. (D) Western blot analysis of cell bound Igs. MCs were exposed to 1% heat-treated rabbit serum for the indicated time intervals in the presence or absence of 3 mM GSH. Cell bound Igs were determined using Western blot analysis. (E, F) Effect of GSH on the cleavage of disulfide bonds in Igs. Heat-treated rabbit serum (1%, E, F) or Thy-1 (1.0 μg/ml, G) was incubated with the indicated concentrations of GSH, NAC or 50 mM DTT for 60 min. After treatment, the samples were separated by SDS page and immunoblotted with an HRP-labeled anti-rabbit or anti–mouse antibody.