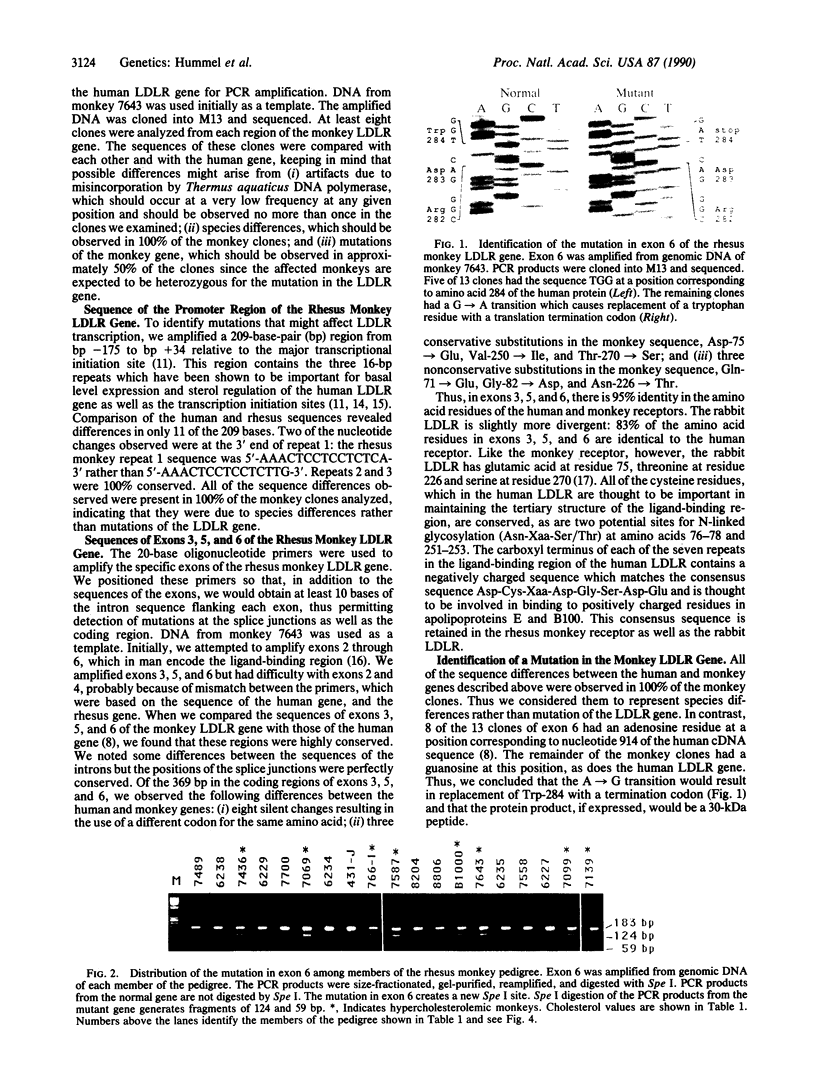
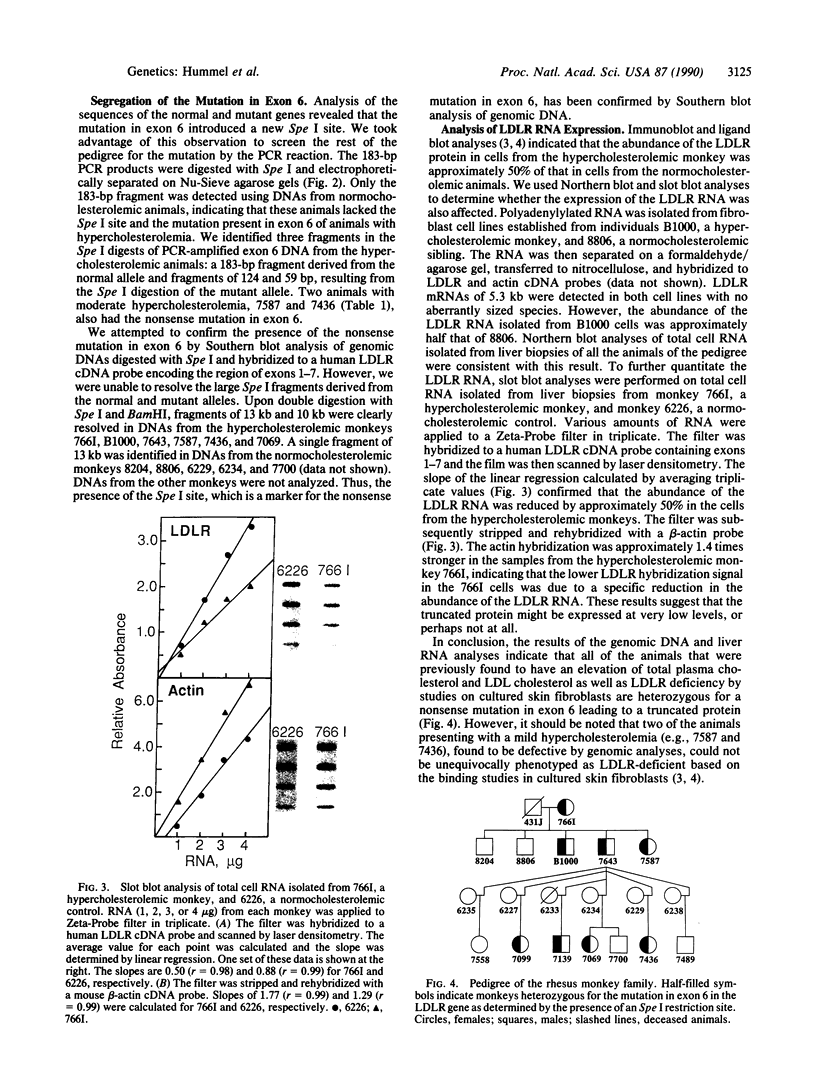
Abstract
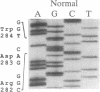
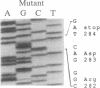
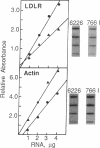
We have recently identified a family of rhesus monkeys with members exhibiting a spontaneous hypercholesterolemia associated with a low density lipoprotein receptor (LDLR) deficiency. By using the polymerase chain reaction, we now show that the affected monkeys are heterozygous for a nonsense mutation in exon 6 of the LDLR gene. This mutation changes the sequence of the codon for amino acid 284 (tryptophan) from TGG to TAG, thereby generating a nonsense codon potentially resulting in a truncated 283-amino acid protein, which needs documentation, however. This G----A mutation also creates a site for the restriction endonuclease Spe I. Using this site as a marker for this nonsense mutation, we have shown that the mutation is present in all of the affected members of the pedigree and absent in unaffected members and that the mutation segregates with the phenotype of spontaneous hypercholesterolemia through three generations. Quantitative analyses of RNA obtained from liver biopsies show that the abundance of the LDLR RNA is also reduced by about 50%. Thus, we have identified a primate model for human familial hypercholesterolemia which will be useful for studying the relationship between the LDLR and lipoprotein metabolism and for assessing the efficacy of diets and drugs in the treatment of human familial hypercholesterolemia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Atweh G. F., Brickner H. E., Zhu X. X., Kazazian H. H., Jr, Forget B. G. New amber mutation in a beta-thalassemic gene with nonmeasurable levels of mutant messenger RNA in vivo. J Clin Invest. 1988 Aug;82(2):557–561. doi: 10.1172/JCI113632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga S. J., Benz E. J., Jr Nonsense mutations in the human beta-globin gene affect mRNA metabolism. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2056–2060. doi: 10.1073/pnas.85.7.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B., Potash M. J., Köhler G. Consequences of frameshift mutations at the immunoglobulin heavy chain locus of the mouse. EMBO J. 1985 Feb;4(2):351–359. doi: 10.1002/j.1460-2075.1985.tb03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Daar I. O., Maquat L. E. Premature translation termination mediates triosephosphate isomerase mRNA degradation. Mol Cell Biol. 1988 Feb;8(2):802–813. doi: 10.1128/mcb.8.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison P. J., Stalenhoef A. F., Humphries S. E. Apolipoprotein CII (apo CII) gene expression defect in an individual with familial apo CII deficiency. Biochem Biophys Res Commun. 1987 Oct 14;148(1):320–328. doi: 10.1016/0006-291x(87)91113-2. [DOI] [PubMed] [Google Scholar]
- Dawson P. A., Hofmann S. L., van der Westhuyzen D. R., Südhof T. C., Brown M. S., Goldstein J. L. Sterol-dependent repression of low density lipoprotein receptor promoter mediated by 16-base pair sequence adjacent to binding site for transcription factor Sp1. J Biol Chem. 1988 Mar 5;263(7):3372–3379. [PubMed] [Google Scholar]
- Oste C. Polymerase chain reaction. Biotechniques. 1988 Feb;6(2):162–167. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Scanu A. M., Khalil A., Neven L., Tidore M., Dawson G., Pfaffinger D., Jackson E., Carey K. D., McGill H. C., Fless G. M. Genetically determined hypercholesterolemia in a rhesus monkey family due to a deficiency of the LDL receptor. J Lipid Res. 1988 Dec;29(12):1671–1681. [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., Russell D. W., Brown M. S., Goldstein J. L. 42 bp element from LDL receptor gene confers end-product repression by sterols when inserted into viral TK promoter. Cell. 1987 Mar 27;48(6):1061–1069. doi: 10.1016/0092-8674(87)90713-6. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Van der Westhuyzen D. R., Goldstein J. L., Brown M. S., Russell D. W. Three direct repeats and a TATA-like sequence are required for regulated expression of the human low density lipoprotein receptor gene. J Biol Chem. 1987 Aug 5;262(22):10773–10779. [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Ciudad C. J., Chasin L. A. Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol Cell Biol. 1989 Jul;9(7):2868–2880. doi: 10.1128/mcb.9.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermann G. The mysteries of lipoprotein(a). Science. 1989 Nov 17;246(4932):904–910. doi: 10.1126/science.2530631. [DOI] [PubMed] [Google Scholar]
- Watanabe Y. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit). Atherosclerosis. 1980 Jun;36(2):261–268. doi: 10.1016/0021-9150(80)90234-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Bishop R. W., Brown M. S., Goldstein J. L., Russell D. W. Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science. 1986 Jun 6;232(4755):1230–1237. doi: 10.1126/science.3010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]