Fig. 3. Changes of the fast conformational dynamics of the hydrophobic core upon nucleotide and pseudosubstrate binding.
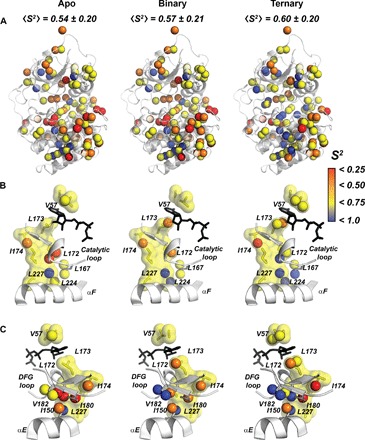
(A) Order parameters of the methyl groups mapped onto the apo, binary (ATPγC-bound), and ternary (ATPγN/PKI5–24) forms of the enzyme, showing an increasing rigidification of the hydrophobic core. (B) Ordering of the C-spine, αF, and the catalytic loop upon nucleotide and PKI binding. (C). Structural details of PKA-C showing the C-spine and the αE helix, highlighting the rigidification of I150, I180, and V182 upon ligand binding and bridging β1-β2 in the N-lobe with β7-β8 in the C-lobe.
