Fig. 4. Synchronization of motions within the hydrophobic core upon nucleotide binding.
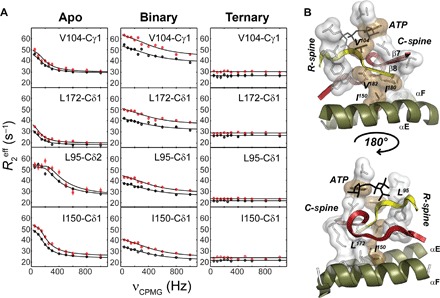
(A) 13C CPMG relaxation dispersion curves carried out at two different magnetic fields (700 MHz, black symbols; 850 MHz, red symbols) for selected residues in the hydrophobic core for the apo, binary (ATPγC-bound), and ternary (ATPγN/PKI5–24) complexes. (B) Mapping of the methyl groups showing conformational dynamics in the C-spine, R-spine, and bridging residues of the hydrophobic core. V104 bridges the R-spine and C-spine together with the adenine ring of ATP, whereas I150 bridges the C-spine to the αE helix.
