Fig. 4. Electrochemical stability of the Li and garnet interface.
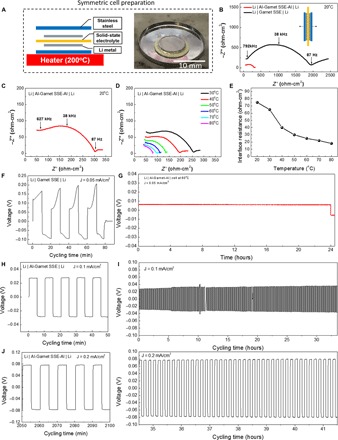
(A) Schematic of the symmetric cell preparation and a digital image of Li metal melting on a garnet SSE. (B and C) Comparison of Nyquist plots of Li | garnet SSE | Li and Li | Al–garnet SSE–Al | Li in the frequency of 1 MHz to 100 mHz at 20°C. (D) Nyquist plots of Li | Al–garnet SSE–Al | Li symmetric cell at various elevated temperatures. (E) The interfacial resistance of the Li | Al–garnet SSE–Al | Li symmetric cell as a function of temperature during heating. (F) Voltage profile depicting the Li plating/striping behavior for the Li | garnet SSE | Li symmetric cell at a current density of 0.05 mA/cm2. The voltage plateau continued to increase each cycle due to the high polarization at the unfavorable Li/SSE interface. The high voltage range reflects the large interfacial resistance for the pristine garnet with Li metal. (G) Li plating of the symmetric Li | Al-garnet-Al | Li cell at 60°C with a current density of 0.05 mA/cm2 for 24 hours. (H to K) Voltage profiles for the Li | Al–garnet SSE–Al | Li symmetric cell at current densities of 0.1 and 0.2 mA/cm2. The voltage plateau remained flat and stable during cycling, which proves that the Li-Al alloy creates a stable interface between the garnet SSE and the Li metal. The low voltage range illustrates the small interfacial resistance in the cell.
