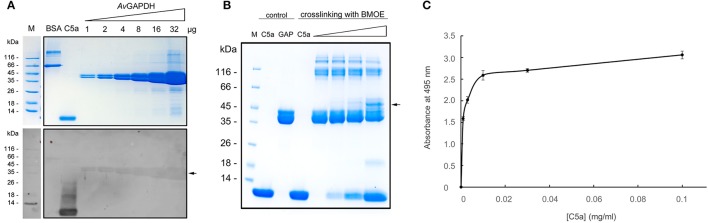
Figure 5.
AvGAPDH interacts with human C5a. (A) Detection of C5a interaction by full-length AvGAPDH in ligand blotting experiments. Top, Coomassie brilliant blue (CBB)-stained SDS-PAGE gel where BSA (negative control), biotinylated C5a (positive control, detectable without added biotinylated C5a), and increasing amounts of pure AvGAPDH (1−32 μg) were separated. Bottom, ligand blotting was conducted after the electrotransfer of the same SDS-PAGE gel shown on top onto nitrocellulose membrane by incubating the membrane with biotinylated C5a and developing the blot with horse radish peroxidase (HRP)-conjugated anti-biotin antibody and luminol. (B) Crosslinking of AvGAPDH and C5a with BMOE shown on a CBB-stained SDS-PAGE gel loaded with mock-treated control samples (C5a and AvGAPDH, GAP) and BMOE-treated crosslinked samples (increasing concentrations of C5a for a fixed concentration of AvGAPDH). The two first lanes with added BMOE represent internal controls for C5a (maximum load) and AvGAPDH (no C5a). In (A) and (B) arrowheads point to the bands where AvGAPDH and C5a co-localize. (C) C5a bound to AvGAPDH immobilized on a polystyrene microtiter plate measured by an ELISA assay. The absorbance at 495 nm is plotted against increasing C5a concentrations. Data represents mean ± SD (standard deviation; N = 3).