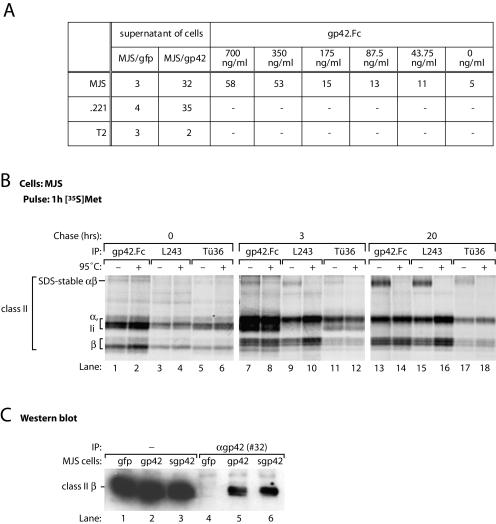
FIG. 3.
s-gp42 is capable of binding to both immature and mature HLA-DR molecules. (A) Culture supernatants of MJS/gp42 cells and control MJS/gfp cells were incubated with HLA class II+ MJS and .221 cells or with HLA class II− T2 cells; for comparison, the binding of gp42.Fc was included. Cell-associated gp42 was detected with antiserum #32 and analyzed by flow cytometry. Mean fluorescence values are depicted; −, not tested. (B) MJS cells were metabolically labeled with [35S]Met for 1 h and chased for 3 or 20 h before lysis. Immunoprecipitation (IP) was performed with s-gp42.Fc or HLA-DR-specific MAbs L243 and Tü36. Immune complexes were incubated in nonreducing sample buffer for 3 h at 37°C (−) or for 5 min at 95°C (+) prior to separation by SDS-12% PAGE. (C) Total lysates of MJS/gp42 cells (lanes 2 and 5), MJS/Kbss-gp42 cells (lanes 3 and 6), and control MJS/gfp cells (lanes 1 and 4) were used directly (lanes 1 to 3) or were subjected to immunoprecipitation with rabbit antiserum #32 against gp42 (lanes 4 to 6). Total lysates and immune complexes were boiled in nonreducing sample buffer, separated by SDS-12% PAGE, and blotted onto polyvinylidene difluoride membranes. Western blots were stained with a MAb specific for HLA-DR β chains (HB10A) and visualized by enhanced chemiluminescence.