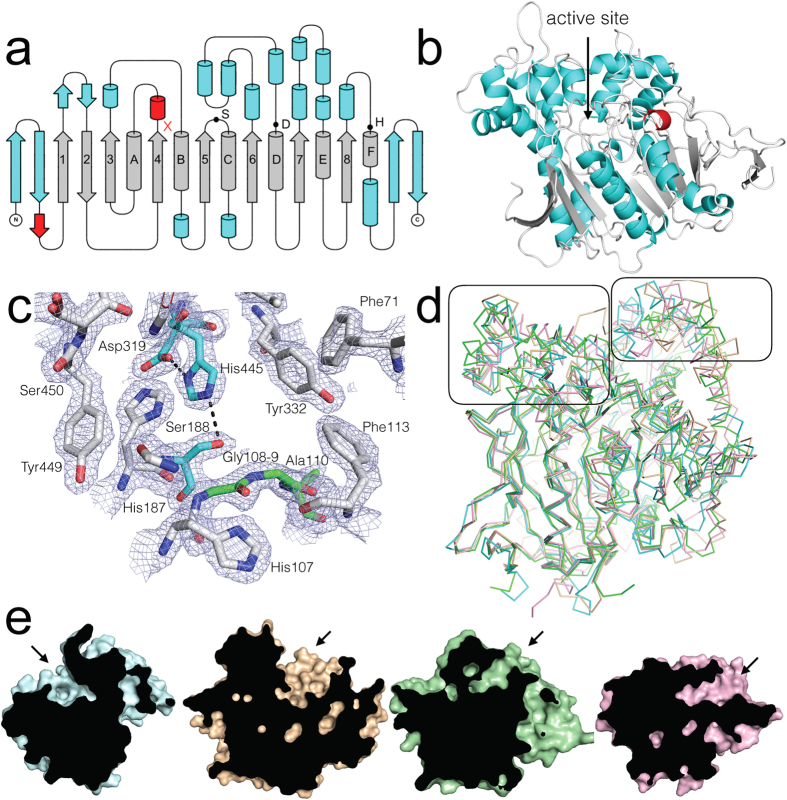
Figure 2. The structure of EST6 from D. melanogaster.
(a) Topology representation of EST6 displaying the conserved α/β-hydrolase fold (grey), secondary structure found in the structurally similar proteins (blue) and unique secondary structure (red). S, D, H represent the Ser188, Asp319 and His445 residues that make up the catalytic triad. The oxyanion hole is located in the loop following sheet 4 (marked by a red x). (b) Cartoon diagram of EST6 with features shown in the topology model similarly coloured. The location of the active site is indicated. (c) The active site of EST6 with 2mFo−dFc electron density contoured at 1.5 σ. The active site serine and histidine from the catalytic triad are coloured cyan, the oxyanion hole (Gly108, Gly109, Ala110) is coloured green. (d) An overlay of EST6 (cyan), LcαE7 (tan; 4FNM), DmAChE (green; 1QO9) and MsJHE (pink; 2FJ0). Conservation of the core β-sheet and conserved α-helices is apparent, but the structures diverge in the region that forms the active site entrance. These regions, either side of the active site, are boxed for clarity. (e) A superposition of EST6, LcαE7, DmAChE and MsJHE, with cut-aways through the middle of the active site. The location of the active site entrance difference between EST6 (cyan) and the other related insect carboxylesterases LcαE7 (tan; 4FNM), DmAChE (green; 1QO9) and MsJHE (pink; 2FJ0).