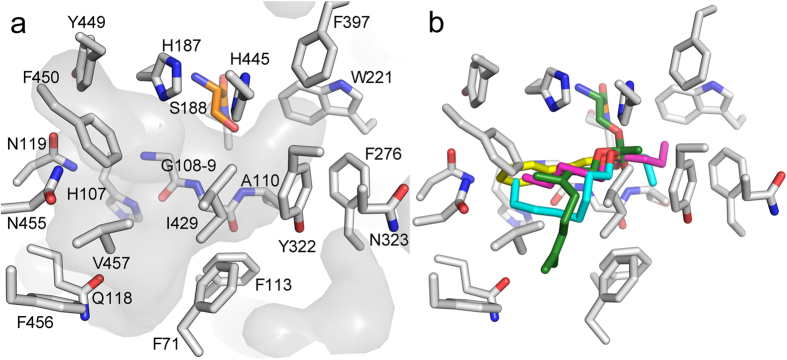
Figure 3. The substrate binding site of EST6.
(a) The surface of the substrate binding site is shaded grey and the residues that comprise the small and large pockets are shown (grey) as is the catalytic serine (orange). The small site consists of Ala110, Trp221, Phe276, Tyr322, Phe397 and His445, and the large site consists of Gln70, Phe71, Phe113, Gly114, Gln118, Asn119, Ile429, Tyr449, Phe450, Asn455, Phe456, and Val457. (b) An overlay of representative acylated enzyme intermediates covalently docked into EST6: the efficiently hydrolyzed substrates pentyl butyrate (magenta), octyl propionate (cyan), geranyl acetate (green) and phenethyl acetate (yellow) all produce acylated intermediates that are accommodated by the substrate binding site.