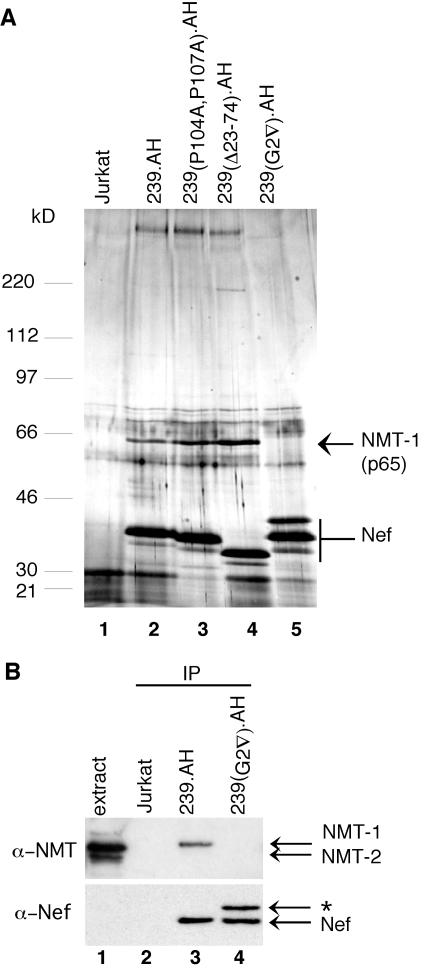
FIG. 1.
Nef associates with NMT-1 in a myristoylation signal-dependent manner. (A) p65 specifically copurifies with SIVmac 239 Nef. Nef and associated proteins were isolated by use of a two-step immunoaffinity purification protocol from detergent extracts prepared from Jurkat T cells expressing AH epitope-tagged wild-type (lane 2) and mutant (lanes 3 to 5) forms of SIVmac 239 Nef. Purification from control puromycin-resistant Jurkat cells that did not express Nef is also shown (lane 1). Eluates were separated by SDS-8 to 17% gradient PAGE, stained with silver nitrate, and analyzed by LC/MS/MS. Bands corresponding to NMT-1 and SIV Nef are indicated. The bands migrating just below Nef likely are Nef degradation products. The positions and molecular masses of protein standards are indicated on the left. (B) Immunoblot analysis identifies Nef-associated p65 as N-myristoyltransferase 1. Immunopurifications from Jurkat T cells expressing wild-type (lane 3) and myristoylation signal-mutated (lane 4) SIVmac 239 Nef and from puromycin-resistant Jurkat T cells that did not express Nef (lane 2) were resolved by SDS-PAGE and analyzed by immunoblotting with α-NMT Ab (upper panel) or α-Nef Ab (bottom panel). Extract from Jurkat cells, which express both the NMT-1 and NMT-2 isoforms, provided a positive control for NMT expression (lane 1). The identity of the α-Nef Ab reactive band indicated with an asterisk is not known.