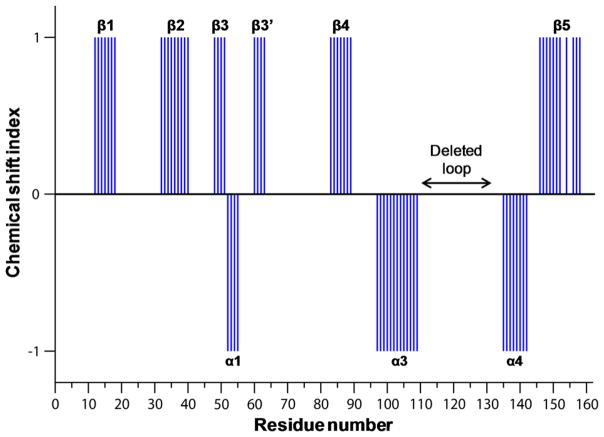
Fig. 3.
Plot of chemical shift index as a function of residue number of AIPL1 FKBPΔloop. Wild type AIPL1 FKBP protein sequence numbering is used here. The deleted loop residues in our AIPL1 FKBPΔloop construct are indicated. The secondary structures obtained from the analyses of the assigned backbone of the AIPL1 FKBPΔloop are also labeled in the figure. Interestingly, the N-terminal α0 helix present in AIP FKBP is absent in AIPL1 FKBPΔloop. Furthermore, the backbone amides of G64-L76 in AIPL1 FKBPΔloop were broad beyond detection, and the corresponding residues in AIP FKBP contain a short loop and an α2 helix.