Abstract
D-galactose induces pancreatic disorder along with aging mouse model. Vitex agnus-castus (VAC) has potential pancreatic protective effect. Hence, this study was designed to evaluate the hypoglycemic and pancreas protective effects of VAC hydroalcoholic extract in D-galactose-induced aging female mice. In the present experimental study, 72 adult female Naval Medical Research Institute (NMRI) mice (weighing 30–35 g) were divided into 6 groups of control, VAC hydroalcoholic extract, D-galactose, D-galactose + VAC hydroalcoholic extract, aged, aged + VAC hydroalcoholic extract. The aged model was prepared by subcutaneous injection of D-galactose for 45 days and, VAC hydroalcoholic extract was gavaged twice a day in the last 7 days. 24 h after the last drug and extract administrations, serum samples and pancreatic tissues were removed to evaluate experimental and histological determinations. Serum glucose level decreased in VAC, D-galactose and, aged-treated groups compared to the control (P < 0.05). Insulin level increased in VAC and decreased in D-galactose and aged VAC-treated mice compared to the control (P < 0.05). Homeostasis model assessment-estimated insulin resistance (HOMA-IR) increased in D-galactose, aging, and VAC hydroalcoholic extract groups (P < 0.05) and, administration of VAC hydroalcoholic extract improved HOMA-IR in D-galactose and aging treated animals. Despite the size of pancreatic islets decreased in aged and D-galactose groups, VAC administration recovered it. Present data showed that VAC hydroalcoholic extract has hypoglycemic and pancreatic protective effects in natural aged and aging model mice.
Key words: Vitex agnus-castus, Pancreas, D-galactose, Aged mouse
INTRODUCTION
Impairments of pancreatic physiological functions including alterations in growth, secretion, and motility have been occurred through the aging process(1). Age-associated diseases such as diabetes are more common in the elderly population. Further, animal model of aging play an important role in drug discovery for aging animal models. One of the important animal models of aging is D-galactose-induced aging mouse model used by different scientific groups(2). D-galactose is a reducing sugar, which can be converted into aldose, hydrogen peroxide (H2O2) and superoxide anions (O2-). These changes are similar to the normal aging process and demonstrate a reduction in antioxidant defense systems such as antioxidant enzyme activity(3). Glucose and lipid metabolism disorders have been considered as important factors contributing to the D-galactose induced aging mouse model(2).
Also, a lot of evidence indicates that free radicals play an important role in the development of diabetes mellitus symptoms such as insulin resistance, impaired glucose tolerance and beta cell dysfunction(4).
Vitex agnus-castus (VAC) commonly known as chaste tree, belongs to the Lamiaceae family of plants and traditionally has been used as a remedy for menstrual problems including premenstrual symptoms and spasmodic dysmenorrheal, insufficient lactation and, acne. VAC has also been used as anticonvulsant, antiepileptic, sedative, and libido reducing plant in the Iranian traditional medicine. Phytochemical analysis of VAC revealed that the fruits, flowers and, leaves of this plant contain flavonoid, iridoids, volatile oils, and tannins(5). It is confirmed that VAC leave extracts has hypoglycemic activity in STZ-induced diabetic rats(6). Also, it has been revealed that D-galactose induces beta cell damage and reduces insulin secretion. Hence, this study was designed to evaluate the hypoglycemic and pancreas protective effects of the VAC hydroalcoholic extract in D-galactose induced aging mouse model.
MATERIALS AND METHODS
Plant extraction
In this experimental study, VAC desiccated fruits were obtained from green grocery of Ahvaz city, Iran and, scientifically authenticated by the Botany Department of Ahvaz Jundishapur University of Medical Science (AJUMS), Ahvaz, Iran. The fruits were powdered and, 250 g of its powder was soaked in 1 L of distilled water-ethanol mixture (70:30) and maintained for 48 h at room temperature. This mixture was filtered and centrifuged at 3000 rpm for 10 min. Then, the supernatant was taken away, dried at 37 °C and, the acquired semi-solid mass was stored at 4 °C until used(7).
Animal preparation
Since this plant has potential effects on aged and post-menopausal symptoms, it was decided to use 72 adult female Naval Medical Research Institute (NMRI) mice (weighing 30-35 g) in present study. The mice obtained from the animal facility of AJUMS, treated according to AJUMS animal care guidelines with an ethics committee grantee ID number (IR. AJUMS. REC. 1394. 724) and kept at 20 ± 4 °C temperature with a 12-h light/ 12-h dark cycle. All animals had free access to tap water and commercial chow ad libitum.
Experimental design
Estrus cycle assessment
Since female steroid hormones can influence variations of serum insulin homeostasis and glucose metabolism through the estrus cycle(8), the same estrus cycle of hormonal synchronization was prepared in female mice. For this purpose, 42 h after injection of 100 μg estradiol valerate dissolved in 0.2 mL olive oil, 50 μg of progesterone was injected intramuscularly. Then, 6 h after the injection of progesterone, vaginal smears were prepared. All smears were fixed on slides in a warm condition, stained with methylene blue (1%) and examined by optical microscope(9,10).
Animals grouping
After induction of the same estrus cycle mice were divided into 6 groups of 12 each including 4 groups of young animals and 2 groups of old mice.
Young animals: control group, received a subcutaneous (s.c) injection of sodium chloride 0.9% as D-galactose solvent for 45 days and same solution was gavaged as VAC solvent twice a day in the last 7 days; VAC hydroalcoholic extract, received sodium chloride 0.9% s.c for 45 days and gavaged VAC extract (600 mg/kg) twice a day in the last 7 days(12); D-galactose, as aging model received D-galactose (500 mg/kg) s.c for 45 days and sodium chloride 0.9% was gavaged twice a day in the last 7 days(11); D-galactose + VAC hydroalcoholic extract, received D-galactose (500 mg/kg) s.c for 45 days and VAC extract (600 mg/kg) was gavaged twice a day in the last 7 days.
Aging animals consisted of 18- to 24-month old mice that received sodium chloride 0.9% s.c for 45 days and same solution as the gavage twice a day in the last 7 days, and aged + VAC hydroalcoholic extract including 18- to 24-month old mice that received sodium chloride 0.9% injection s.c for 45 days and were gavaged VAC extract (600 mg/kg) twice a day in the last 7 days.
Experimental measurement
Twenty four hours after the last D-galactose and extract administrations, animals were sacrificed under deep anesthesia by intraperitoneal injection of ketamine-xylazine mixture (70:10 mg/kg) and blood samples were obtained by cardiac puncture. Blood samples were centrifuged at 3000 rpm for 15 min to harvest their serum contents. Then, all samples maintained at −20 °C until hormonal assessment(13). Glucose was measured by biochemical assay kits (Pars Azmoon, Iran) and serum insulin levels were determined by ELISA assays kits (Monobind, USA) (The sensitivity of hormone detection per assay tube was 0.182 μIU/mL). Further, homeostasis model assessment-estimated insulin resistance (HOMA-IR) was calculated according to the following formula(14).
HOMA-IR = fasting insulin (μIU/dL) × fasting glucose (mg/dL)/ 405.
Histological assessment
Mouse pancreatic tissue was removed and fixed in 10% formalin solution. Then, tissues were dehydrated in graded alcohol concentrations and embedded in paraffin. Sections of 4-6 μm were prepared and stained with hematoxylin & eosin (H&E). Six with hematoxylin & eosin (H&E). Six microscopic H&E stained slides per animal were examined for assessment of histological changes such as cellular swelling and diameters of islets which assessed by using Motic Images Plus 2.0 image analysis software. Finally, a blind method was used for slides reading(15,16).
Statistical assessment
All data are reported as mean ± SEM and were analyzed using SPSS software with one-way ANOVA which was then followed by post hoc LSD tests. P values less than 0.05 was assumed statistically significant.
RESULTS
Hypoglycemic effects of Vitex agnus-castus extract
VAC hydroalcoholic extract decreased serum glucose levels in both VAC and D-galactose VAC-treated groups when compared to the controls (P < 0.05). Also, the same effect was observed in D-galactose VAC-treated mice in comparison with D-galactose group (P < 0.05). Moreover, administration of VAC extract reduced glucose serum levels in aged treated mice compared to aged and control groups (P < 0.01, Fig. 1). While serum insulin levels increased in VAC and aging groups, this hormone significantly decreaseed in aged VAC-treated mice when compared to the control animals (P < 0.05).
Fig. 1.
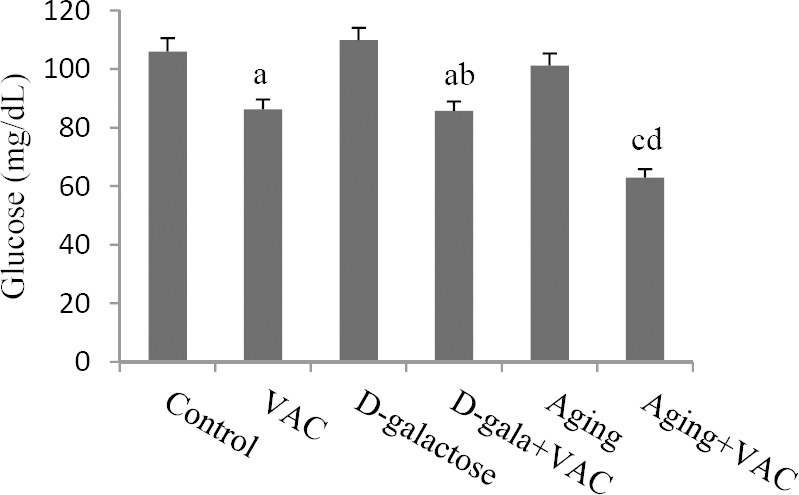
Effect of Vitex agnus-castus (VAC) hydroalcoholic extract on serum glucose levels. aDenotes significant difference (P < 0.05) compared to the control, bdenotes significant difference (P < 0.01) compared to the D-galactose, cdenotes significant difference (P < 0.01) compared to the control, ddenotes significant difference (P < 0.01) compared to the aging animals.
Similarly, VAC extract administration reduced serum insulin levels in aged treated mice as appose to aged group (P < 0.01, Fig. 2). However, HOMA-IR, as an insulin resistance index, increased in D-galactose, aged and VAC groups; this parameter decreased in D-galactose and aged VAC-treated mice compared with control (P < 0.05). Further, the insulin resistance index showed a significant decrease in D-galactose VAC-treated mice as compared to D-galactose group (P < 0.05). Similar effects were observed in aged VAC-treated mice compared to aged group (P < 0.01, Fig. 3).
Fig. 2.
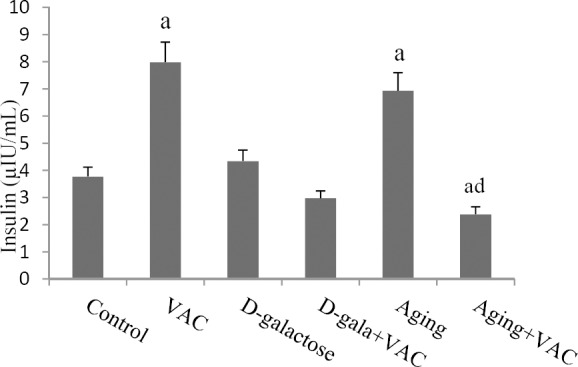
Effect of Vitex agnus-castus (VAC) hydroalcoholic extract on serum insulin levels. aDenotes significant difference (P < 0.05) compared to the control, ddenotes significant difference (P < 0.01) compared to the aging mice.
Fig. 3.
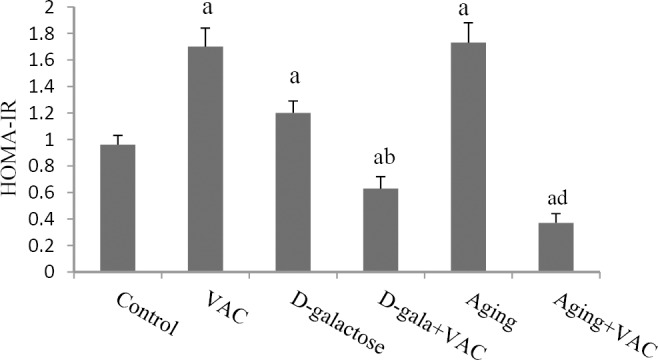
Effect of Vitex agnus-castus (VAC) hydroalcoholic extract on homeostasis model assessment-estimated insulin resistance (HOMA-IR). aDenotes significant difference (P < 0.05) compared to the control, bdenotes significant difference (P < 0.05) compared to the D-galactose, ddenotes significant difference (P < 0.01) compared to the aging group.
Histopathological and histomorphometric evaluation
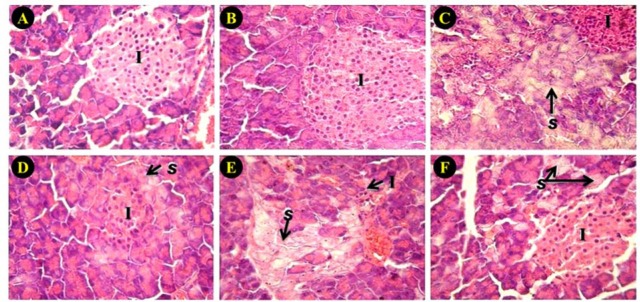
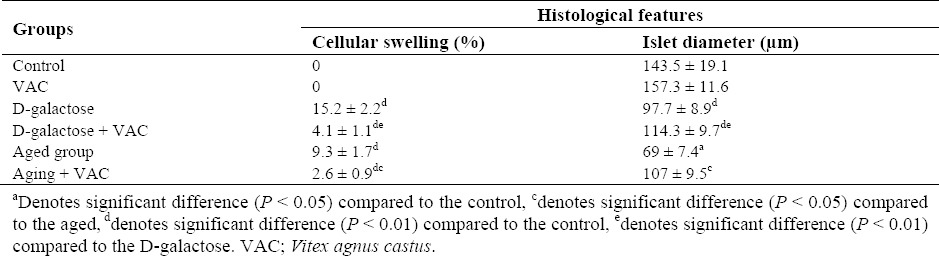
All pancreatic sections revealed a normal appearance in control and VAC hydro-alcoholic extract groups. In natural aged and D-galactose group, the diameter of islets were significantly decreased (P < 0.05). Cell swelling in pancreatic acini was significantly increased in these animals and these alterations were significantly attenuated by VAC extract. Corresponding data are shown in Fig. 4 and Table 1.
Fig. 4.
Effect of Vitex agnus-castus (VAC) hydroalcoholic extract on pancreas histology. (A) control, (B) VAC hydro alcoholic extract, (C) D-galactose, (D) D-galactose + VAC hydro alcoholic extract, (E) aged, (F) aged + VAC hydro alcoholic extract (H&E stain, × 400). (I) infiltration of inflammatory cells; (S) swelling cytoplasm.
Table 1.
Effect of Vitex agnus-castus (VAC) extract on pancreatic histopathology of normal, D-galactose and aged mice.
DISCUSSION
The present study indicates that VAC induces hyperinsulinemia and hypoglycemic effects in the normal adult mice. Various studies showed that liver and exocrine tissues have the capacity of extra pancreatic insulin production and storage(17). Del Prato, et al. provided that the main cause of fasting hypoglycemia with hyperinsulinemia is the inhibition of hepatic glucose production concomitant with peripheral tissue insulin resistance(18). Also, Service, et al. demonstrated that beta cell hyper function can produce hypoglycemia through the excessive insulin secretion, which lead to insulin resistance(19). Hence, present results in normal mice indicate that VAC extract may affect through the beta cell hyper function to increase insulin secretion but, there was not enough opportunity for insulin receptors to utilize this hormone and leads to increase HOMA-IR. Further, the glucose carrier isoform found in peripheral tissues is regulated by insulin. Insulin stimulation promotes the fusion of these carriers with the plasma membrane and accelerates glucose transport. On the other hand, because limited numbers of carrier molecules are present in the membrane, raising the solute concentration sufficiently will saturate all the carrier molecules so that no more glucose can be bound and this limits the maximal rate of transport(20). Hence, it could be suggested that despite of the increased serum insulin levels in normal VAC-treated mice, maximal transport of glucose has been occurred in them and this event leads to increase HOMA-IR levels. Gu, et al. reported that although the fasting blood glucose (FBG) were within the normal range in aged animals the fasting insulin level increased in them. This finding suggested that old rats increased insulin secretion to maintain FBG in normal levels. Therefore, hyperinsulinemia was an indirect indication of insulin resistance. There is a lot of evidence that age and free radicals play an important role in the development of insulin resistance, impaired glucose tolerance and, beta cell dysfunction(21). Hence, compared to D-galactose-induced aging model, present data suggest that natural aged animals showed less beta cell dysfunction via increased more serum insulin levels. Regarding insulin resistance, several complications such as accumulation of lipid metabolism intermediates, increased muscle atrophy and decrease in glucose carrier isoform have been occurred through the aged process(22). Further, Stella, et al. study indicated that VAC extract decreases serum level of glucose in diabetic rats via modifying of glucose utilization, stimulates the regeneration of beta cells, and enhances glucose transport to the peripheral tissue(6). So, present results indicate that the hypoglycemic effects of VAC extract in D-galactose and aged treated animals may be occurred through the improvement of mentioned age-related criteria and it may be more effective on glucose transport carrier or insulin resistance, but future study is required to clarify the exact mechanism of this event.
In histological assessment we found that the diameter of islets was decreased by D-galactose which may relate to a decrease in insulin secretion. The interstitial fibrosis progression has a main factor in the distortion of exocrine and endocrine pancreas histoarchitecture that is related to vascular, ductal insular alterations. Further, the progressive enhancement of the pancreatic adipose tissue was noted in the aging rats frequently which is associated with the natural degenerative event of aging(23). Cell swelling was observed in pancreatic acini. It is well known that cell swelling can lead to cell death. Treatment of animals with the VAC hydroalcoholic extract showed an increase in diameter of pancreatic islets and a decrease in acinar cell damages. Thus, VAC may suppress apoptosis in both D-galactose and aging animals. Anti apoptotic effect of VAC has been shown in previous studies(24,25).
CONCLUSION
Present data revealed that VAC hydroalcoholic extract has a hypoglycemic and pancreatic protective effect in natural aged and aging model treated animals. Extract treatment improved hyperglycemia, insulin resistance and beta cell reduction induced by aging. Further studies are required to determine the exact mechanism of VAC on age-related changes in the pancreas.
ACKNOWLEDGMENTS
This study is labeled Health Research Institute, Diabetes Research Center Research Project No. D-9414 and financially supported by Health Research Institute, Diabetes Research Center of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
REFERENCES
- 1.Watanabe H, Saito H, Rychahou PG, Uchida T, Evers BM. Aged is associated with decreased pancreatic acinar cell regeneration and phosphatidylinositol 3-kinase/Akt activation. Gastroenterology. 2005;128(5):1391–1404. doi: 10.1053/j.gastro.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Zhou YY, Ji XF, Fu JP, Zhu XJ, Li RH, Mu CK, et al. Gene transcriptional and metabolic profile changes in mimetic aged mice induced by D-galactose. PLoS One. 2015;10(7):e0132088. doi: 10.1371/journal.pone.0132088. doi:10.1371/journal.pone.0132088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahangarpour A, Oroojan AA, Heidari H. Effects of Exendin-4 on LH, FSH, testosterone and sperm count of aged mice model induced by D-galactose. World J Mens Health. 2014;32(3):176–183. doi: 10.5534/wjmh.2014.32.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asgary S, Naderi GA, Shams Ardekani MR, Sahebkar A, Airin A, Aslani S, et al. Inhibition of protein glycation by essential oils of branchlets and fruits of Juniperus communis subsp. hemisphaerica. Res Pharm Sci. 2014;9(3):179–185. [PMC free article] [PubMed] [Google Scholar]
- 5.Daniele C, Thompson Coon J, Pittler MH, Ernst E. Vitex agnus castus: a systematic review of adverse events. Drug Saf. 2005;28(4):319–332. doi: 10.2165/00002018-200528040-00004. [DOI] [PubMed] [Google Scholar]
- 6.Stella J, Krishnamoorthy P, Mohamed AJ. Hypoglycemic effect of Vitex agnus castus in streptozotocin induced diabetic rats. Asian J Biochem Pharm Res. 2011;2:206–212. [Google Scholar]
- 7.Ahangarpour A, Oroojan AA, Heidari H, Ghaedi E, Taherkani R. effects of hydro-alcoholic extract from arctium lappa l. (burdock) root on gonadotropins, testosterone, and sperm count and viability in male mice with nicotinamide/ streptozotocin-induced type 2 diabetes. Malays J Med Sci. 2015;22(2):25–32. [PMC free article] [PubMed] [Google Scholar]
- 8.Morimoto S, Cerbón MA, Alvarez-Alvarez A, Romero-Navarro G, Díaz-Sánchez V. Insulin gene expression pattern in rat pancreas during the estrous cycle. Life Sci. 2001;68(26):2979–2985. doi: 10.1016/s0024-3205(01)01100-6. [DOI] [PubMed] [Google Scholar]
- 9.Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. The mouse in biomedical research. 2nd edition. Vol. 3. California: American College of Laboratory Animal Medicine Series; 2007. pp. 97–103. [Google Scholar]
- 10.Hoseini E, Forouzan Far M, Paye Dar A. The effect of hydroalcoholic extract of purslane (Portulaca oleracea L) on serum concentration of esterogen, progesterone, prolactin and gonadotropins in mature female rats. J Shahrekord Univ Med Sci. 2013;15(5):12–21. [Google Scholar]
- 11.Ho SC, Liu JH, Wu RY. Establishment of the mimetic aged effect in mice caused by D-galactose. Biogerontology. 2003;4(1):15–18. doi: 10.1023/a:1022417102206. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim N, Shalaby A, Farag R, Elbaroty G, Nofal S, Hassan E. Gynecological efficacy and chemical investigation of Vitex agnus-castus L. fruits growing in Egypt. Nat Prod Res. 2008;22(6):537–546. doi: 10.1080/14786410701592612. [DOI] [PubMed] [Google Scholar]
- 13.Lee EJ, Silva SM, Simões Mde J, Montero EF. Effect of N-acetylcysteine in liver ischemia-reperfusion injury after 30% hepatectomy in mice. Acta Cir Bras. 2012;27(4):346–349. doi: 10.1590/s0102-86502012000400011. [DOI] [PubMed] [Google Scholar]
- 14.Sharma SB, Nasir A, Prabhu KM, Murthy PS, Dev G. Hypoglycaemic and hypolipidemic effect of ethanolic extract of seeds of Eugenia jambolana in alloxan-induced diabetic rabbits. J Ethnopharmacol. 2003;85(2-3):201–206. doi: 10.1016/s0378-8741(02)00366-5. [DOI] [PubMed] [Google Scholar]
- 15.Khorsandi L, Mirhoseini M, Mohamadpour M, Orazizadeh M, Khaghani S. Effect of curcumin on dexamethasone-induced testicular toxicity in mice. Pharm Biol. 2013;51:206–212. doi: 10.3109/13880209.2012.716854. [DOI] [PubMed] [Google Scholar]
- 16.Salahshoor MR, Khashiadeh M, Roshankhah S, Kakabaraei S, Jalili C. Protective effect of crocin on liver toxicity induced by morphine. Res Pharm Sci. 2016;11(2):120–129. [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima H, Fujimiya M, Matsumura K, Nakahara T, Hara M, Chan L. Extra pancreatic insulin-producing cells in multiple organs in diabetes. Proc Natl Acad Sci USA. 2004;101(8):2458–2463. doi: 10.1073/pnas.0308690100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Prato S, Riccio A, Vigili de Kreutzenberg S, Dorella M, Avogaro A, Marescotti MC, et al. Mechanisms of fasting hypoglycemia and concomitant insulin resistance in insulinoma patients. Metabolism. 1993;42(1):24–29. doi: 10.1016/0026-0495(93)90167-m. [DOI] [PubMed] [Google Scholar]
- 19.Service FJ, Natt N, Thompson GB, Grant CS, van Heerden JA, Andrews JC, et al. Noninsulinoma pancreatogenous hypoglycemia: a novel syndrome of hyperinsulinemic hypoglycemia in adults independent of mutations in Kir6.2 and SUR1 genes. J Clin Endocrinol Metab. 1999;84:1582–1589. doi: 10.1210/jcem.84.5.5645. [DOI] [PubMed] [Google Scholar]
- 20.Blaustein MP, Kao JPY, Matteson DR. Cellular physiology and neurophysiology. 2nd ed. New York: Elsevier Inc; 2014. pp. 116–118. [Google Scholar]
- 21.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aged. 2007;2(2):219–236. [PMC free article] [PubMed] [Google Scholar]
- 22.dos Santos JM, Benite-Ribeiro SA, Queiroz G, Duarte JA. The effect of age on glucose uptake and GLUT1 and GLUT4 expression in rat skeletal muscle. Cell Biochem Funct. 2012;30(3):191–197. doi: 10.1002/cbf.1834. [DOI] [PubMed] [Google Scholar]
- 23.Riccillo FL, Bracamonte MI, Cónsole GM, Gómez Dumm CL. Histomorphological and quantitative immunohistochemical changes in the rat pancreas during aged. Biocell. 2004;28(2):127–134. [PubMed] [Google Scholar]
- 24.Hajdú Z, Hohmann J, Forgo P, Martinek T, Dervarics M, Zupkó I, et al. Diterpenoids and flavonoids from the fruits of Vitex agnus-castus and antioxidant activity of the fruit extracts and their constituents. Phytother Res. 2007;21(4):391–394. doi: 10.1002/ptr.2021. [DOI] [PubMed] [Google Scholar]
- 25.Law BN, Ling AP, Koh RY, Chye SM, Wong YP. Neuroprotective effects of orientin on hydrogen peroxide-induced apoptosis in SH-SY5Ycells. Mol Med Rep. 2014;9(3):947–954. doi: 10.3892/mmr.2013.1878. [DOI] [PubMed] [Google Scholar]