Abstract
Dengue virus is an emerging global health threat. The major envelope glycoprotein, E, mediates viral attachment and entry by membrane fusion. Antibodies that bind but fail to neutralize noncognate serotypes enhance infection. We have determined the crystal structure of a soluble fragment of the envelope glycoprotein E from dengue virus type 3. The structure closely resembles those of E proteins from dengue type 2 and tick-borne encephalitis viruses. Serotype-specific neutralization escape mutants in dengue virus E proteins are all located on a surface of domain III, which has been implicated in receptor binding. While antibodies against epitopes in domain I are nonneutralizing in dengue virus, there are neutralizing antibodies that recognize serotype-conserved epitopes in domain II. The mechanism of neutralization for these antibodies is probably inhibition of membrane fusion. Our structure shows that neighboring glycans on the viral surface are spaced widely enough (at least 32 Å) that they can interact with multiple carbohydrate recognition domains on oligomeric lectins such as DC-SIGN, ensuring maximum affinity for these putative receptors.
Dengue virus, a member of the flavivirus family, imposes one of the largest social and economic burdens of any mosquito-borne viral pathogen (12). There is no specific treatment for infection, and control of dengue virus by vaccination has proved elusive (5). The presence of multiple dengue virus serotypes in circulation leads to an increase in pathogenesis (10). The principal reason for this enhancement is thought to be as follows (see reference 14 for a review). Structural differences at the viral surface in different serotypes mean that neutralizing antibodies raised against one serotype are likely to bind other serotypes with deceased affinity. As the affinity of an antibody decreases, so does its ability to neutralize the virus. Lower-affinity antibodies will fail to neutralize, but they may still be present on virus particles in sufficient numbers to facilitate infection of cells bearing immunoglobulin G (IgG) Fc receptors (15, 31). Alternatively, some high-affinity antibodies that are neutralizing at high titers can enhance infection at subneutralizing concentrations by facilitating cell uptake of virus of the cognate serotype (3, 16, 38).
Flaviviruses other than dengue virus are important human pathogens, including yellow fever, West Nile, tick-borne encephalitis (TBE), and Japanese encephalitis viruses (5). Three structural proteins (C, M, and E) and a lipid bilayer package the positive-strand RNA genome (30). The core nucleocapsid protein, C, assembles with RNA on the cytosolic face of the endoplasmic reticulum membrane. The assembling core buds through the endoplasmic reticulum membrane, thereby acquiring an envelope that contains the major envelope glycoprotein, E, and the so-called precursor membrane protein, PrM. The particle passes through the secretory pathway, where a furin-like protease cleaves PrM to M in a late trans-Golgi compartment. The cleavage, which removes most of the ectodomain of PrM, releases a constraint on E and primes the particle for low-pH-triggered membrane fusion. Uncleaved, immature particles are not fusion competent (5, 30). In addition to forming the outer protein shell (24), E also binds a receptor on the cell surface. Receptor binding directs the virion to the endocytic pathway. E responds to the reduced pH of an endosome by conformational rearrangement (36). The conformational change induces fusion of the viral and host-cell membranes, allowing the viral genome to enter the cytoplasm.
It is still unclear what receptor dengue virus recognizes on the cell surface. Moreover, the virus may recognize different receptors in human (host) and mosquito (vector) cells (18). It has been proposed that patches of positively charged residues on the surface of domain III bind to negatively charged heparan sulfate on the cell surface (6, 18). Many cell types express heparan sulfate, however, and a more specific protein receptor is thought to be required to target dengue virus to permissive cell types, immature dendritic cells in particular (51). Glycans on the virus surface have recently been implicated in receptor binding by the finding that DC-SIGN, a mannose-specific, oligomeric C-type lectin on the cell surface, is essential for productive infection of dendritic cells (39, 48). These glycans must belong to E, as it is the only glycoprotein on the surface of the mature virion. DC-SIGN is specific to immature dendritic cells, which have been proposed to be the primary target cells upon introduction of dengue virus into the bloodstream (51).
The structures of the E protein ectodomains from dengue virus type 2 (DEN-2) and from TBE virus have been determined by X-ray crystallography (35, 44). We now report the structure of a soluble fragment (residues 1 to 393) of the E protein from DEN-3. This fragment contains all but about 50 residues of the E-protein ectodomain (Fig. 1). It closely resembles its homologs from DEN-2 and TBE viruses in its dimeric structure and in the details of its protein fold. The clustering of serotype-specific neutralization escape mutants in domain III, which has been implicated in receptor binding (6, 18, 44), suggests that a likely mechanism of neutralization for antibodies against these epitopes is inhibition of cellular attachment. The structure also shows that neighboring glycans on the viral surface are spaced in such a way that oligomeric lectins such as DC-SIGN could bind tightly through multiple attachment points.
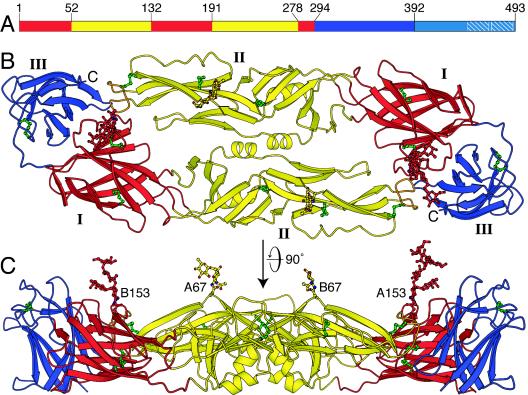
FIG. 1.
Structure of the sE dimer of dengue virus E sE in the mature virus particle. (A) The three domains of dengue virus sE. Domain I is red, domain II is yellow, and domain III is blue. A 53-residue stem segment links the stably folded sE fragment with the C-terminal transmembrane anchor. (B) The DEN-3 sE dimer viewed along its twofold symmetry axis. (C) The sE dimer viewed perpendicular to its twofold axis. The two glycans on residues 67 and 153 of the two subunits (A and B) of the dimer are labeled.
MATERIALS AND METHODS
Expression and purification of DEN-3 sE.
Soluble E protein (sE) from DEN-3 (42) was expressed and purified at Hawaii Biotech, Inc. (Aiea, Hawaii). The protein was expressed in Drosophila melanogaster Schneider 2 cells (obtained from American Type Culture Collection) with a pMtt vector (GlaxoSmithKline) containing the DEN-3 (strain CH53489) PrM and E genes (nucleotides 437 to 2113), as previously described (20). The resulting PrM-E preprotein is processed during secretion to yield sE, which was purified from the cell culture medium by immunoaffinity chromatography, as previously described (8). Purified sE was concentrated to 12 g liter−1 in Amicon Ultra centrifugal filters (Millipore).
Crystallization and data collection.
Crystals were grown at 20°C by hanging-drop vapor diffusion by mixing equal volumes of protein solution and the following reservoir solution: 15% polyethylene glycol 4000, 0.1 M Tris-HCl (pH 8.5), and 0.2 M lithium sulfate. Crystals grew as rods in space group P212121 with the following cell dimensions: a = 52.9 Å, b = 68.63 Å, and c = 270.2 Å. The asymmetric unit contains two molecules of sE. Crystals were transferred to a cryoprotective solution of 15% polyethylene glycol 4000, 0.1 M Tris-HCl (pH 8.5), 0.2 M lithium sulfate, and 30% glycerol before being frozen in liquid nitrogen. Data were collected at 100 K on beamline F1 of the Cornell High Energy Synchrotron Source (CHESS) at Cornell University, Ithaca, N.Y. The data were processed with HKL2000 (43). Data collection statistics are presented in Table 1.
TABLE 1.
Crystallographic data and refinement statistics
| Data set (space group) | P212121 |
|---|---|
| Molecules per asymmetric unit | 2 |
| Cell edges a, b, c (Å) | 52.9, 68.6, 270.2 |
| Resolution range (Å) | 20-3.6 |
| % Completenessa | 92 (81) |
| I/σ(I)a | 12.3 (2.6) |
| Rmerge (%)a | 12.0 (53.8) |
| Unique reflections | 12,090 |
| Rcrystb | 0.279 |
| Rfreec | 0.324 |
| Avg B factor (Å2) | |
| Protein (chains A and B) | 73.6, 74.1 |
| Water molecules | 41.7 |
| Rmsdd bond length (Å) | 0.009 |
| Rmsd bond angle (°) | 1.532 |
| Rmsd bonded B factor (Å2) | |
| Main chain | 2.65 |
| Side chain | 3.45 |
| Ramachandran plot (%) | |
| Favored | 71.1 |
| Allowed | 28.2 |
| Generous | 0.7 |
| Disallowed | 0 |
Numbers in parentheses are for the highest-resolution shell, 3.73 to 3.60 Å.
Rcryst, Σhkl‖Fobs| − |〈Fcalc〉‖/Σhkl|Fobs|.
Rfree, Rcryst with 5% of Fobs sequestered before refinement.
Rmsd, root mean square difference.
Structure determination.
The crystal structure of DEN-3 E was determined by molecular replacement with a monomer of the DEN-2 E structure (35) (Protein Data Bank code 1OAN) as the search model. Two monomers were placed sequentially with AMoRe (40). The atomic coordinates of the three domains in each monomer were then refined as rigid bodies with the Crystallography & NMR System (CNS) (4). Amino acids in the model were mutated from the DEN-2 to the DEN-3 sequence, and the model was rebuilt with O (23) based on 2Fo—Fc and Fo—Fc Fourier maps. Coordinates were refined against data between a 20- and 3.7-Å resolution by simulated annealing with torsion-angle dynamics with CNS and then rebuilt with O in iterative cycles. Later cycles included restrained refinement of B factors for individual atoms and energy minimization against a maximum-likelihood target with CNS. In the final refinement cycles, carried out with REFMAC5 (50), the rigid-body motion of the protein molecules in the crystal was taken into account in terms of three tensors: one for translation (T), one for libration (L), and one for correlations of libration and translation (S) (46). This TLS refinement was alternated with cycles of restrained positional and B-factor refinement against a maximum likelihood target. Strict twofold noncrystallographic symmetry restraints were used throughout refinement. The final model contains residues 1 to 392, 14 water molecules, and two simple, fucosylated glycans per protein chain on residues 67 and 153, respectively. The four glycans in the model each contain between one and six ordered sugar rings. Refinement statistics are presented in Table 1. The stereochemical quality of the atomic model was checked and validated with PROCHECK (26).
Accession numbers.
The atomic coordinates of the DEN-3 sE dimer have been deposited in the Protein Data Bank under accession code 1uzg. Structure factors were deposited under accession code r1uzgsf.
RESULTS
Molecular architecture of the DEN-3 sE dimer.
The amino acid sequences of DEN-2 strain PR159S1 and DEN-3 strain CH53489 E proteins are 68% identical. As expected at this level of sequence identity, the soluble fragment of DEN-3 E that we crystallized, DEN-3 sE, adopts the same three-dimensional fold as DEN-2 sE (35). Each sE monomer consists of three domains (Fig. 1A). Domain I, an eight-stranded β-barrel, organizes the structure. Two long insertions between pairs of β-strands in domain I form the elongated domain II, which bears the fusion loop at its tip. Domain II contains 12 β-strands and two α-helices. Domain III is an IgC-like module, with 10 β-strands. In solution and in the crystals, two monomers of sE assemble head to tail to form a dimer, with the long axis of domain II roughly perpendicular to the dyad axis and the fusion loop buried at the dimer interface (Fig. 1B).
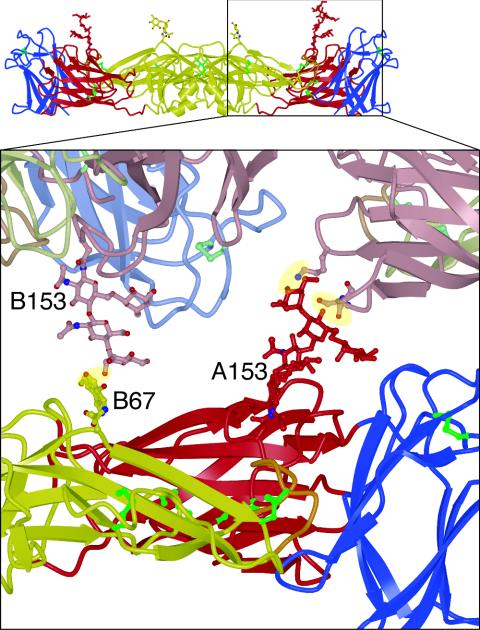
Dengue viruses have two conserved N-linked glycosylation sites at Asn-67 and Asn-153. The glycosylation site at Asn-153 is conserved in most flaviviruses, while the glycosylation site at Asn-67 appears to be unique to dengue viruses and is absent in other class 2 enveloped viruses. The loop bearing the glycan on Asn-153 is two residues shorter in DEN-3 than in the other dengue virus serotypes. Both glycans have been implicated in cellular attachment and viral entry (13, 19, 39, 48). Ordered sugars are visible at both glycosylation sites in the DEN-3 sE structure, indicating that both are utilized in Drosophila cells, as is also the case of DEN-2 sE expressed in Drosophila cells (35). DEN-2 E harvested from virus grown in Aedes albopictus C6/36 cells, however, has been reported to lack glycosylation at Asn-153 (22). In our DEN-3 structure, the glycans on Asn-67 and Asn-153 form crystal contacts with either glycan or protein residues from molecules related by the crystal symmetry, hence contributing to crystallization (Fig. 2). These contacts explain why relatively large portions of the glycans obey the crystal symmetry. An α1-6-linked fucose is visible on the first N-acetylglucosamine of both the Asn-67 glycan (on the A chain only) and the Asn-153 glycan. The sugar chain is disordered beyond the first N-acetylglucosamine of the Asn-67 glycan, but up to six sugar rings are visible in the electron density for the Asn-153 glycan of the A chain. They were modeled as follows: Asn→GlcNAc(Fucα1→6)β1→4GlcNAc→Manβ1→4(Manα1 →6)α1→3Man; that is, two N-acetylglucosamines with a fucose on the first N-acetylglucosamine and three mannoses linked to the second N-acetylglucosamine through the central mannose (Fig. 1C). This structure (excluding the fucose) corresponds to the pentasaccharide mannose 3 (Man3), which is the common core of all N-linked oligosaccharides. Analysis of the carbohydrate composition of human immunodeficiency virus gp120 overexpressed in Drosophila Schneider 2 cells shows that the glycans consist of simple and high-mannose oligosaccharides (25) rather than the more complex carbohydrates found on mammalian proteins (reviewed in reference 1). Indeed, Drosophila Schneider 2 cells have a high level of endogenous mannosidase activity, which can result in trimming of the full nine-mannose structure of high-mannose glycans to Man3 structures. Both DEN-2 and DEN-3 sEs are resistant to deglycosylation by endoglycosidase H (which is specific for structures with five or more mannoses) but can be completely deglycosylated with either peptide N-glycosidase F or endoglycosidase D (data not shown), suggesting that the glycans on dengue virus sE are efficiently trimmed to simple Man3 structures during expression in Schneider 2 cells. In support of this notion, there is no evidence for glycan residues beyond the Man3 core in any of the glycans in the DEN-2 or DEN-3 crystal structures.
FIG. 2.
Contacts involving the glycans of sE between molecules related by the crystal symmetry. A closeup of the sE dimer (in red, yellow, and blue for domains I, II, and III, respectively) viewed from the side (inset) shows that both glycans form crystal contacts with an adjacent molecule in the crystal (shown in lighter colors). Mannose residues in the glycan on Asn-153 of chain A form hydrogen bonds with the main chain and side-chain oxygen atoms of Ser-16 and the side-chain amine of Lys-36. The N-acetylglucosamine linked to Asn-67 of chain B forms a hydrogen bond with a mannose in the glycan on Asn-153 of chain B of a neighboring molecule in the crystal.
Comparison to the structure of the DEN-2 sE dimer.
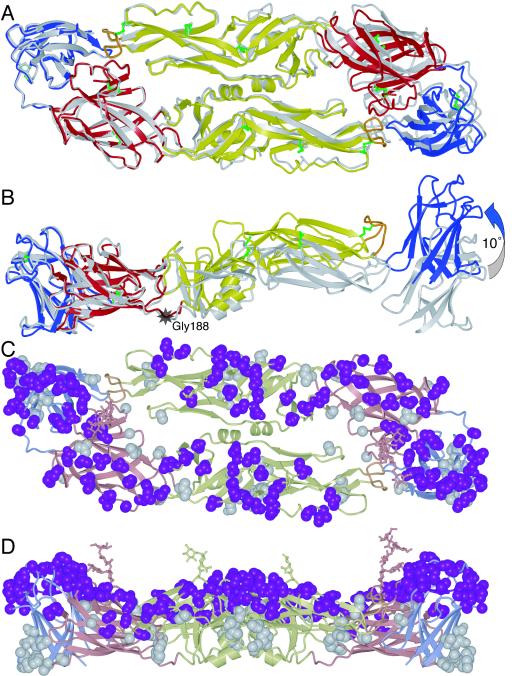
The greatest difference between the DEN-2 (35) and DEN-3 sE structures is in the relative orientation of domains I and II. This difference is most apparent when domain I of a DEN-3 sE monomer is superimposed on domain I of a DEN-2 monomer: the orientations of domain II in each structure are then separated by a 10° rotation at about a point near Gly-188 of DEN-3 (Fig. 3A and B). The altered domain orientation translates into a difference in backbone atom positions of 13 Å at the fusion loop at the tip of domain II and of up to 18 Å at the far end of domain III of the other monomer in the dimer (Fig. 3B). Variations in the relative orientations of domain I and domain II of up to 5° have been observed as a result of a hinge motion around Gly-188 in different crystal forms of DEN-2 (35) and TBE sE (F. A. Rey and S. C. Harrison, unpublished work), suggesting that the sE dimer is somewhat flexible in solution. The 10° difference in the relative orientations of domains I and II of DEN-2 and DEN-3 represents a significantly larger shift, however. If it reflects a real structural difference between the two serotypes rather than conformational flexibility of sE, there could be slight differences in the surface features of DEN-2 and DEN-3 virions.
FIG. 3.
Structure of the DEN-3 sE dimer superimposed on the DEN-2 sE dimer, using domain I from one monomer as the reference, viewed along the twofold symmetry axes (A) and perpendicular to the twofold axes (B). The greatest difference between the two structures is a ∼10° rotation of domain II relative to domain I about a point near Gly-188 (indicated by a grey star). (C) The DEN-3 sE dimer with residues that are not conserved in DEN-2 is shown in space-filling representation. Residues that are exposed on the viral surface are in magenta; residues that are not exposed are in grey. (D) The same structure as in panel C but viewed perpendicular to the twofold axis.
The individual domains of sE in DEN-2 and DEN-3 have a high degree of structural similarity, with root mean square deviations for main chain atoms of 0.78 Å for domain I, 0.73 Å for domain II, and 0.85 Å for domain III. Only three loops adopt substantially different conformations. The loop (residues 153 to 158) that follows the second glycosylation site is two residues shorter in DEN-3. The solvent-exposed loop (residues 167 to 170) between β-strands in domain I also has a different conformation in the two serotypes, largely because Pro-166 in DEN-2 is replaced at the equivalent position in DEN-3 by serine, a conformationally more flexible residue. The third loop with substantial differences in main chain positions (up to 3 Å) spans residues 336 to 346 in DEN-3. Two glycine residues at positions 340 and 342 in DEN-3 replace a leucine and a lysine, respectively. The increased flexibility of glycine allows the loop to reach a conformation in DEN-3 that would have caused strain with the DEN-2 amino acid sequence.
Just over two-thirds of the residues in DEN-2 and DEN-3 sE are identical. Of the residues that are not identical, about one-third have similar length and polarity; the remaining 86 unconserved residues differ substantially in charge, polarity, length, or conformational variability. These 86 residues are distributed fairly evenly throughout the sE structure, although there are none in the area around the fusion loop. They tend to cluster instead in the loops of domain III and in a loop in middle of domain II formed by residues 219 to 234 (Fig. 3C and D). Of the 86 unconserved residues, 56 will be exposed on the viral surface, based on TBE sE fitting into a structure of the entire DEN-2 virion at 9.5 Å resolution, as derived by electron cryomicroscopy (52). All but 5 of the remaining 30 unconserved residues are exposed on the sE protein surface but are not expected to be accessible from outside the virion. Residues 59, 63, 143, 219, and 337 are unconserved despite being buried in the protein core, but none of these residues have charged side chains. The different binding affinities of neutralizing antibodies to dengue virus serotypes are likely to be due to variations in residues that are exposed on the viral surface. We therefore conclude, based on the DEN-3 sE structure, that only the 56 unconserved residues exposed on the viral surface (or a subset thereof) are the most important contributors to differential antibody binding.
DISCUSSION
Implications for immune recognition of different dengue virus serotypes.
Structural features on the surface of the virion determine the specificity with which a given antibody will bind. Differences in the surface landscape of the four dengue virus serotypes mean that neutralizing antibodies raised against one serotype are likely to bind other serotypes with deceased affinity. Antibodies with a sufficiently low binding affinity may fail to neutralize the virus but may still be present in sufficient numbers on virions that they can induce cell uptake through interaction with IgG Fc receptors (15, 31). Dengue virus is able to use these molecules as surrogate receptors. By the same mechanism, some high-affinity antibodies that neutralize virus of a certain serotype at high titers can increase cell uptake of the same serotype at sufficiently low antibody concentrations (3, 16, 38). The resulting enhancement of viral infection may cause more acute pathologies such as hemorrhagic fever (15, 37). Antibody-dependent enhancement of dengue virus infection is a problem particularly upon reinfection with a second serotype, because antibodies against the first serotype may bind but not neutralize the second serotype. Indeed, the circulation of multiple serotypes in Cuba and southeast Asia where dengue fever is hyperendemic has coincided with the emergence of more severe dengue pathologies, dengue hemorrhagic fever and dengue shock syndrome, but also provides synergies between different serotypes. These synergies allow multiple strains to coexist in human populations and to generate complex and persistent epidemic cycles (7, 10, 41). It is therefore important to determine which surface features on the dengue virion are responsible for immune recognition in the different serotypes.
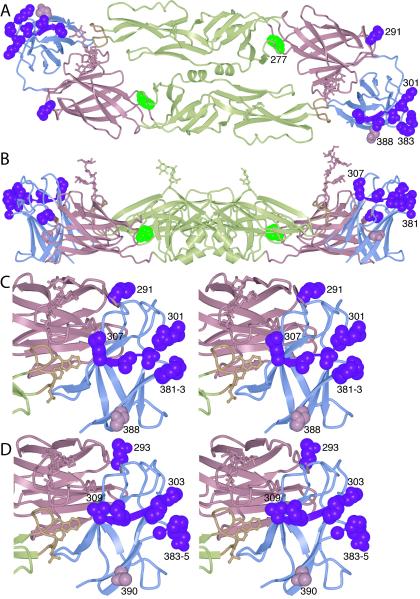
From the data presented above, we conclude that the differential antibody binding to different dengue serotypes is likely to be due to a variation in a subset of the 56 unconserved residues that are exposed on the viral surface. These residues cover the majority of the known epitopes responsible for escape from neutralization by monoclonal antibodies. The escape mutants that map to serotype-specific residues are not evenly distributed throughout the structure. Instead, they lie exclusively in domain III (Fig. 4). They include residue 291 (2), residue 305 (29), and residues 381 to 383 (17, 18). Residue 388 is also unconserved and exposed on the viral surface. An asparagine at this position appears to cause increased incidences of hemorrhagic fever, while charged residues reduce virulence (28). Residue 388 may therefore participate, directly or indirectly, in receptor binding. The localization of all these mutants in domain III is significant, because domain III has been proposed to bind a cell-surface receptor (see below). The clustering of serotype-specific neutralization escape mutants in domain III suggests that a likely mechanism of neutralization for antibodies against these epitopes is inhibition of receptor binding and cellular attachment.
FIG. 4.
Antibody neutralization-escape mutants in dengue virus. (A) Three serotype-specific epitopes have been reported: residue 291 (2), residues 301 to 307 (29), and residues 381 to 383 (17, 18). Side chains in all three epitopes are shown on the DEN-3 E structure in magenta in space-filling representation. Changes at residue 388 (in pink), also unconserved and exposed, correlate with changes in virulence (28). Phe-277 (in green) is conserved in the dengue virus serotypes, but its mutation to serine during passaging in cell culture leads to escape from neutralization by IgM M10 (2, 27). (B) A side view of panel A is shown. (C) Stereoscopic view of a close-up of DEN-3 domain III, showing the four proposed serotype-specific epitopes. The orientation is halfway between the orientations used in the models shown in panels A and B, and the close-up is of the copy of domain III shown on the right in panels A and B. (D) The same view as in panel C is shown, but of DEN-2 (and with the homologous residues highlighted).
While most well-characterized neutralizing antibodies recognize epitopes in domain III, some (both neutralizing and nonneutralizing) have epitopes on one of the other two domains of E (Fig. 4A and B; Table 2). Monoclonal antibodies raised against epitopes in domain I are type specific (that is, affected by residues not conserved across dengue serotypes) but nonneutralizing (33). At the domain I-domain II boundary, a Phe277Ser mutation causes escape from neutralization by IgM M10 and alters the pH threshold for fusion (27). Phe277, conserved in all dengue serotypes (2), is accessible from the viral surface (Fig. 4A). It is located in the kl-hairpin region of domain II, which has a role in the conformational rearrangement that drives membrane fusion (35, 36, 44). Other antibodies to epitopes in domain II are also group reactive; that is, they recognize residues that are strictly conserved in the different serotypes (33). These antibodies appear to neutralize the virus by inhibiting membrane fusion (33, 45), rather than by blocking attachment. Indeed, West Nile virus can attach and enter endosomes in the presence of polyclonal antibodies, but membrane fusion and viral entry are both inhibited (11).
TABLE 2.
Dengue virus mutationsa
| Mutation | Serotype | MAb | Phenotype | Explanation |
|---|---|---|---|---|
| T(Q)293I | DEN-1 | IgM M17 | More virulent and hemagglutinating at pH 5.8-7 | At a hinge between domains I and III; may rigidify the hinge, requiring lower pH for conformational switch (2) |
| K307E | DEN-2 | IgG G8D11 | None | IgG G8D11 may interfere with receptor binding (29) |
| E311G | DEN-2 | IgM 6B2 | None | IgM 6B2 may interfere with receptor binding (32) |
| E383G P384E/D/N G385K/S/A | DEN-2 | IgG 3H5 | Reduced mouse neurovirulence. | IgG 3H5 may interfere with receptor binding (17), probably in mosquito cells and not in human cells (18) |
Mutations in dengue virus that lead to escape from neutralization by monoclonal antibodies, resulting phenotypes, and possible explanations based on the structures of sE of DEN-2 and -3. Residue numbers refer to DEN-2. For unconserved residues, the residue type in DEN-2 is listed in parentheses before the residue number. Residues that are unconserved among dengue serotypes are in boldface type.
Implications for receptor binding.
Several lines of evidence suggest that domain III binds a cellular receptor. Recombinant DEN-2 domain III expressed alone interferes with infection by blocking virion adsorption to both mammalian and mosquito cells (18). Furthermore, a loop formed by residues 381 to 384 is responsible for the serotype-specific attachment of domain III to mosquito (but not mammalian) cells (18). In tick-borne viruses such as the TBE virus, this loop is four residues shorter and forms a tight β-turn (44). Many of the residues implicated as determinants of host range, tropism, or virulence in various flaviviruses are located in domain III (21, 44); see Table S1 in the supplemental materials for examples in dengue virus). The widespread occurrence of Ig modules in cell adhesion proteins is also consonant with the notion that domain III participates in attachment to a cellular receptor. From the structure of DEN-2 sE in the postfusion conformation (36), none of the neutralization-escape mutations in domain III are expected to interfere with the conformational rearrangement that accompanies membrane fusion, and they do not alter the pH threshold of fusion (Table 2). The most likely mechanism of neutralization by antibodies recognizing epitopes on domain III is therefore inhibition of cellular attachment. The efficiency of the inhibition of viral attachment by these antibodies is relatively low (55 to 60%), however, suggesting that other factors may contribute to neutralization (45).
It is still unclear what molecules domain III recognizes on a cell surface. Moreover, dengue virus may recognize different receptors on human and mosquito cells (18). There are currently no candidate protein receptors, but it has been proposed that patches of basic residues on the surface of domain III bind to heparan sulfate on the cell surface, which carries a net negative charge (6, 18, 49). The DEN-3 sE structure shows that only the first of two putative heparan binding motifs (6) in domain I of sE forms a cluster of positively charged residues on the viral surface. The cluster consists of Arg-188, His-280, Lys-282, Arg-284, Lys-286, Lys-289, and Lys-293, all of which are conserved as basic residues across dengue virus serotypes but not in TBE virus. Examination of the DEN-2 and DEN-3 sE structures reveals that another basic patch on the viral surface is formed in the middle of domain II near the dimer interface by residues Lys-58, Lys-64, Lys-128, and Lys-200. The basic cluster is larger in DEN-2, as it also includes Lys-89, Lys-122, and Lys-123. Most of the residues in this cluster are not conserved in TBE virus. Thus, the positively charged patches of lysines and arginines on domains I and II could in principle contribute to heparan sulfate binding. Many cell types express heparan sulfate, however, and a more specific protein receptor is thought to be required to target dengue virus to permissive cell types—immature dendritic cells in particular (51).
Glycans on the dengue virus surface were recently implicated in receptor binding when DC-SIGN, a mannose-specific, C-type lectin on the cell surface of immature dendritic cells, was found to be essential for productive infection (39, 48). These glycans must belong to E, as it is the only glycoprotein on the surface of the mature virion. Immature dendritic cells have been proposed to be the primary target cells upon introduction of dengue virus into the bloodstream (51). Dengue virus infection of human dendritic cells is inhibited by anti-DC-SIGN antibodies and by the soluble ectodomain of DC-SIGN (39, 48). Flavivirus infection requires clathrin-mediated uptake into the endocytic pathway and acidification in the endosome (5, 39). The cytoplasmic tail of DC-SIGN contains two sorting signals for the endocytic pathway (47); these sorting signals could be responsible for targeting dengue virus to the endosome. DC-SIGN is a type II integral membrane protein. Its extracellular domain has a stalk and a C-terminal C-type carbohydrate recognition domain (CRD). The stalk causes the protein to form tetramers through an α-helical coiled-coil interaction (34). The CRD selectively recognizes endogenous high-mannose oligosaccharides (9, 34), and it binds glycopeptides bearing two such oligosaccharides with a 5- to 25-fold-higher affinity (34). This oligovalency may be important for recognition of dengue virus because dengue virus E has two glycosylation sites at Asn-67 and Asn-153, unlike the envelope proteins of other flaviviruses, which have only the latter. Furthermore, the binding of a substrate is enhanced substantially if the spacing between the glycans is sufficient for each glycan to reach a CRD in the DC-SIGN tetramer (34). The evolution of a second glycosylation site in dengue virus E that is appropriately spaced from the first site may thus have allowed E to bind DC-SIGN with sufficient affinity for it to function as a receptor. Indeed, based on our structure of DEN-3 sE and a fit of TBE E into the electron cryomicroscopy structure of DEN-2 virions (52), the spacing between any two neighboring glycans on the viral surface is at least 32 Å. This distance should be sufficient to span between two CRDs in the DC-SIGN tetramer, based on the crystal structure of the CRD-mannose complex (9) and on the predicted structure of the DC-SIGN tetramer (34). All four CRDs in a DC-SIGN tetramer should thus be able to bind a glycan on the dengue virion. The absence of glycosylation on Asn-153 in DEN-2 E under certain conditions (22), however, suggests that certain dengue virus strains or serotypes may be viable with only a single glycan per E monomer, on Asn-67.
Conclusions.
Comparison of the prefusion structures of E from DEN-2 and DEN-3 shows that the differences are, as anticipated, relatively subtle. Our analysis of the locations of the known type- and group-specific epitopes poses the following questions. Are antibodies that recognize epitopes conserved among serotypes (e.g., those in domain II) also cross-neutralizing at a reasonable titer, or are deviations from conservation at the periphery of the antibody binding site sufficient to prevent adequate interaction of an antibody with any but the serotype against which it was raised? If there is some degree of cross-neutralization, would increasing the titer of such antibodies by a suitable immunization strategy lead to useful cross-protection, or would the risk of antibody-dependent enhancement outweigh potential benefits? Answers to these and related questions will require careful analysis of the spectrum of epitopes recognized by circulating antibodies in patient sera, the changes in this spectrum as a function of time, and the correlation of the results with the response to subsequent infection by a different dengue virus serotype. The E-protein structures will allow such data to be interpreted directly in terms of well-defined molecular surfaces.
Supplementary Material
Acknowledgments
This work was supported by NIH grants CA-13202 and AI-57159 to S.C.H. S.C.H. is a Howard Hughes Medical Institute Investigator.
We thank staff at the F1 Beamline of the Cornell High Energy Synchrotron Source (CHESS) at Cornell University, Ithaca, N.Y., and John Roehrig for comments on the manuscript.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Altmann, F., E. Staudacher, I. B. Wilson, and L. Marz. 1999. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj. J. 16:109-123. [DOI] [PubMed] [Google Scholar]
- 2.Beasley, D. W., and J. G. Aaskov. 2001. Epitopes on the dengue 1 virus envelope protein recognized by neutralizing IgM monoclonal antibodies. Virology 279:447-458. [DOI] [PubMed] [Google Scholar]
- 3.Brandt, W. E., J. M. McCown, M. K. Gentry, and P. K. Russell. 1982. Infection enhancement of dengue type 2 virus in the U-937 human monocyte cell line by antibodies to flavivirus cross-reactive determinants. Infect. Immun. 36:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brünger, A. T., P. D. Adams, G. M. Clore, W. L. DeLano, P. Gros, R. W. Grosse-Kunstleve, J. S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography & NMR System: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54:905-921. [DOI] [PubMed] [Google Scholar]
- 5.Burke, D. S., and T. P. Monath. 2001. Flaviviruses, p. 1043-1125. In D. M. Knipe, P. M. Howley, D. E. Griffin, S. R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 6.Chen, Y., T. Maguire, R. E. Hileman, J. R. Fromm, J. D. Esko, R. J. Linhardt, and R. M. Marks. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3:866-871. [DOI] [PubMed] [Google Scholar]
- 7.Cummings, D. A., R. A. Irizarry, N. E. Huang, T. P. Endy, A. Nisalak, K. Ungchusak, and D. S. Burke. 2004. Travelling waves in the occurrence of dengue haemorrhagic fever in Thailand. Nature 427:344-347. [DOI] [PubMed] [Google Scholar]
- 8.Cuzzubbo, A. J., T. P. Endy, A. Nisalak, S. Kalayanarooj, D. W. Vaughn, S. A. Ogata, D. E. Clements, and P. L. Devine. 2001. Use of recombinant envelope proteins for serological diagnosis of Dengue virus infection in an immunochromatographic assay. Clin. Diagn. Lab. Immunol. 8:1150-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinberg, H., D. A. Mitchell, K. Drickamer, and W. I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163-2166. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson, N., R. Anderson, and S. Gupta. 1999. The effect of antibody-dependent enhancement on the transmission dynamics and persistence of multiple-strain pathogens. Proc. Natl. Acad. Sci. USA 96:790-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gollins, S. W., and J. S. Porterfield. 1986. A new mechanism for the neutralization of enveloped viruses by antiviral antibody. Nature 321:244-246. [DOI] [PubMed] [Google Scholar]
- 12.Gubler, D. J. 2002. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 10:100-103. [DOI] [PubMed] [Google Scholar]
- 13.Guirakhoo, F., A. R. Hunt, J. G. Lewis, and J. T. Roehrig. 1993. Selection and partial characterization of dengue 2 virus mutants that induce fusion at elevated pH. Virology 194:219-223. [DOI] [PubMed] [Google Scholar]
- 14.Halstead, S. B. 1988. Pathogenesis of dengue: challenges to molecular biology. Science 239:476-481. [DOI] [PubMed] [Google Scholar]
- 15.Halstead, S. B., and E. J. O'Rourke. 1977. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 146:201-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halstead, S. B., C. N. Venkateshan, M. K. Gentry, and L. K. Larsen. 1984. Heterogeneity of infection enhancement of dengue 2 strains by monoclonal antibodies. J. Immunol. 132:1529-1532. [PubMed] [Google Scholar]
- 17.Hiramatsu, K., M. Tadano, R. Men, and C. J. Lai. 1996. Mutational analysis of a neutralization epitope on the dengue type 2 virus (DEN2) envelope protein: monoclonal antibody resistant DEN2/DEN4 chimeras exhibit reduced mouse neurovirulence. Virology 224:437-445. [DOI] [PubMed] [Google Scholar]
- 18.Hung, J. J., M. T. Hsieh, M. J. Young, C. L. Kao, C. C. King, and W. Chang. 2004. An external loop region of domain III of dengue virus type 2 envelope protein is involved in serotype-specific binding to mosquito but not mammalian cells. J. Virol. 78:378-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung, S. L., P. L. Lee, H. W. Chen, L. K. Chen, C. L. Kao, and C. C. King. 1999. Analysis of the steps involved in dengue virus entry into host cells. Virology 257:156-167. [DOI] [PubMed] [Google Scholar]
- 20.Ivy, J., E. Nakano, and D. Clements. December 2000. U.S. patent 6,165,477.
- 21.Jennings, A. D., C. A. Gibson, B. R. Miller, J. H. Mathews, C. J. Mitchell, J. T. Roehrig, D. J. Wood, F. Taffs, B. K. Sil, S. N. Whitby, J. E. Whitby, T. P. Monath, P. D. Minor, P. G. Sanders, and A. D. T. Barrett. 1994. Analysis of a yellow fever virus isolated from a fatal case of vaccine-associated human encephalitis. J. Infect. Dis. 169:512-518. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, A. J., F. Guirakhoo, and J. T. Roehrig. 1994. The envelope glycoproteins of dengue 1 and dengue 2 viruses grown in mosquito cells differ in their utilization of potential glycosylation sites. Virology 203:241-249. [DOI] [PubMed] [Google Scholar]
- 23.Jones, T. A., J. Y. Zou, S. W. Cowan, and M. Kjeldgaard. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47:110-119. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn, R. J., W. Zhang, M. G. Rossmann, S. V. Pletnev, J. Corver, E. Lenches, C. T. Jones, S. Mukhopadhyay, P. R. Chipman, E. G. Strauss, T. S. Baker, and J. H. Strauss. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwong, P. D., R. Wyatt, E. Desjardins, J. Robinson, J. S. Culp, B. D. Hellmig, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1999. Probability analysis of variational crystallization and its application to gp120, the exterior envelope glycoprotein of type 1 human immunodeficiency virus (HIV-1). J. Biol. Chem. 274:4115-4123. [DOI] [PubMed] [Google Scholar]
- 26.Laskowski, R. A., M. W. MacArthur, D. S. Moss, and J. M. Thornton. 1993. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26:283-291. [Google Scholar]
- 27.Lee, E., R. C. Weir, and L. Dalgarno. 1997. Changes in the dengue virus major envelope protein on passaging and their localization on the three-dimensional structure of the protein. Virology 232:281-290. [DOI] [PubMed] [Google Scholar]
- 28.Leitmeyer, K. C., D. W. Vaughn, D. M. Watts, R. Salas, I. Villalobos de Chacon, C. Ramos, and R. Rico-Hesse. 1999. Dengue virus structural differences that correlate with pathogenesis. J. Virol. 73:4738-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, B., C. R. Parrish, J. M. Murray, and P. J. Wright. 1994. Localization of a neutralizing epitope on the envelope protein of dengue virus type 2. Virology 202:885-890. [DOI] [PubMed] [Google Scholar]
- 30.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe, P. M. Howley, D. E. Griffin, S. R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 31.Littaua, R., I. Kurane, and F. A. Ennis. 1990. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J. Immunol. 144:3183-3186. [PubMed] [Google Scholar]
- 32.Lok, S. M., M. L. Ng, and J. Aaskov. 2001. Amino acid and phenotypic changes in dengue 2 virus associated with escape from neutralisation by IgM antibody. J. Med. Virol. 65:315-323. [DOI] [PubMed] [Google Scholar]
- 33.Megret, F., J. P. Hugnot, A. Falconar, M. K. Gentry, D. M. Morens, J. M. Murray, J. J. Schlesinger, P. J. Wright, P. Young, M. H. Van Regenmortel, and V. Deubel. 1992. Use of recombinant fusion proteins and monoclonal antibodies to define linear and discontinuous antigenic sites on the dengue virus envelope glycoprotein. Virology 187:480-491. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell, D. A., A. J. Fadden, and K. Drickamer. 2001. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 276:28939-28945. [DOI] [PubMed] [Google Scholar]
- 35.Modis, Y., and S. C. Harrison. 2003. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 100:6986-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2004. Structure of the dengue virus envelope protein after membrane fusion. Nature 427:313-319. [DOI] [PubMed] [Google Scholar]
- 37.Mongkolsapaya, J., W. Dejnirattisai, X. N. Xu, S. Vasanawathana, N. Tangthawornchaikul, A. Chairunsri, S. Sawasdivorn, T. Duangchinda, T. Dong, S. Rowland-Jones, P. T. Yenchitsomanus, A. McMichael, P. Malasit, and G. Screaton. 2003. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 9:921-927. [DOI] [PubMed] [Google Scholar]
- 38.Morens, D. M., S. B. Halstead, and N. J. Marchette. 1987. Profiles of antibody-dependent enhancement of dengue virus type 2 infection. Microb. Pathog. 3:231-237. [DOI] [PubMed] [Google Scholar]
- 39.Navarro-Sanchez, E., R. Altmeyer, A. Amara, O. Schwartz, F. Fieschi, J. L. Virelizier, F. Arenzana-Seisdedos, and P. Despres. 2003. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 4:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navaza, J. 2001. Implementation of molecular replacement in AMoRe. Acta Crystallogr. D Biol. Crystallogr. 57:1367-1372. [DOI] [PubMed] [Google Scholar]
- 41.Nisalak, A., T. P. Endy, S. Nimmannitya, S. Kalayanarooj, U. Thisayakorn, R. M. Scott, D. S. Burke, C. H. Hoke, B. L. Innis, and D. W. Vaughn. 2003. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am. J. Trop. Med. Hyg. 68:191-202. [PubMed] [Google Scholar]
- 42.Osatomi, K., and H. Sumiyoshi. 1990. Complete nucleotide sequence of dengue type 3 virus genome RNA. Virology 176:643-647. [DOI] [PubMed] [Google Scholar]
- 43.Otwinowski, Z., and W. Minor. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276:307-326. [DOI] [PubMed] [Google Scholar]
- 44.Rey, F. A., F. X. Heinz, C. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 45.Roehrig, J. T., R. A. Bolin, and R. G. Kelly. 1998. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology 246:317-328. [DOI] [PubMed] [Google Scholar]
- 46.Schomaker, V., and K. N. Trueblood. 1968. On the rigid-body motion of molecules in crystals. Acta Crystallogr. B. 24:63-76. [Google Scholar]
- 47.Soilleux, E. J., R. Barten, and J. Trowsdale. 2000. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J. Immunol. 165:2937-2942. [DOI] [PubMed] [Google Scholar]
- 48.Tassaneetrithep, B., T. H. Burgess, A. Granelli-Piperno, C. Trumpfheller, J. Finke, W. Sun, M. A. Eller, K. Pattanapanyasat, S. Sarasombath, D. L. Birx, R. M. Steinman, S. Schlesinger, and M. A. Marovich. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thullier, P., C. Demangel, H. Bedouelle, F. Megret, A. Jouan, V. Deubel, J. C. Mazie, and P. Lafaye. 2001. Mapping of a dengue virus neutralizing epitope critical for the infectivity of all serotypes: insight into the neutralization mechanism. J. Gen. Virol. 82:1885-1892. [DOI] [PubMed] [Google Scholar]
- 50.Winn, M. D., G. N. Murshudov, and M. Z. Papiz. 2003. Macromolecular TLS refinement in REFMAC at moderate resolutions. Methods Enzymol. 374:300-321. [DOI] [PubMed] [Google Scholar]
- 51.Wu, S. J., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816-820. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, W., P. R. Chipman, J. Corver, P. R. Johnson, Y. Zhang, S. Mukhopadhyay, T. S. Baker, J. H. Strauss, M. G. Rossmann, and R. J. Kuhn. 2003. Visualization of membrane protein domains by cryo-electron microscopy of dengue virus. Nat. Struct. Biol. 10:907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.