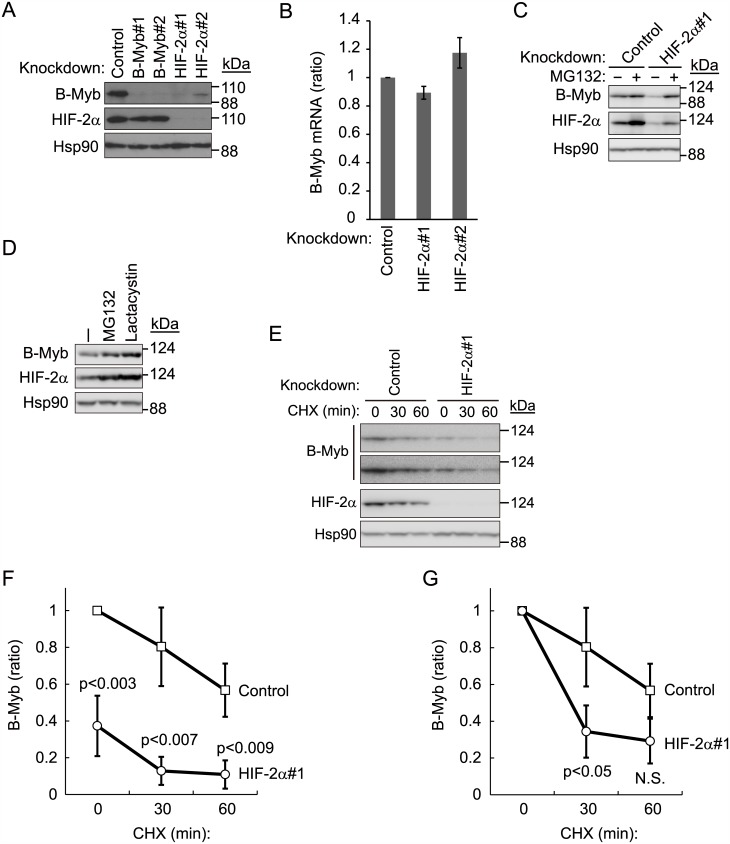
Fig 1. Knockdown of HIF-2α downregulates B-Myb in pVHL-deficient 786-O cells.
(A) Knockdown of HIF-2α downregulates B-Myb protein levels in pVHL-deficient 786-O cells. Cell lysates of control-, B-Myb-, or HIF-2α-knockdown 786-O cell lines were immunoblotted with anti-B-Myb or anti-HIF-2α antibodies to determine protein levels of B-Myb and HIF-2α, respectively. Hsp90 was used as loading control. Representative data from three independent experiments are shown. (B) Knockdown of HIF-2α does not affect B-Myb mRNA levels. Total RNA was isolated from control- or HIF-2α-knockdown 786-O cells and analyzed by quantitative RT-PCR analysis. Data represent the mean ± SD of three independent experiments. (C) B-Myb downregulation by HIF-2α knockdown in pVHL-deficient cells is proteasome-dependent. Control or HIF-2α-knockdown 786-O cells were cultured in the presence or absence of MG132 (10 μM for 6 h) and subjected to immunoblotting with anti-B-Myb or anti-HIF-2α antibodies. Hsp90 was used as loading control. Representative data from three independent experiments are shown. (D) Stabilization of HIF-2α and B-Myb by MG132 and lactacystin. 786-O cells were cultured in the presence or absence of MG132 (10 μM for 6 h) or lactacystin (5 μM for 6 h) and immunoblotted with anti-B-Myb or anti-HIF-2α antibodies. Hsp90 was used as loading control. Representative data from three independent experiments are shown. (E) Stability of B-Myb with or without HIF-2α. Control 786-O cells or HIF-2α knockdown cells were exposed to cycloheximide (CHX, 50 μg/ml) for 30 or 60 min. The lysates were subjected to western blot with antibodies against B-Myb (short and long exposure are shown), HIF-2α, or Hsp90. Hsp90 was used as loading control. Representative data from three independent experiments are shown. (F) The intensities of the B-Myb bands in (D) were normalized to those of the corresponding Hsp90 bands and plotted as ratio of the normalized value against control cells at 0 min. Data are presented as the mean ± SD of three independent experiments. (G) The intensities of B-Myb bands in (D) at 0 min were set as 1.