Figure 4.
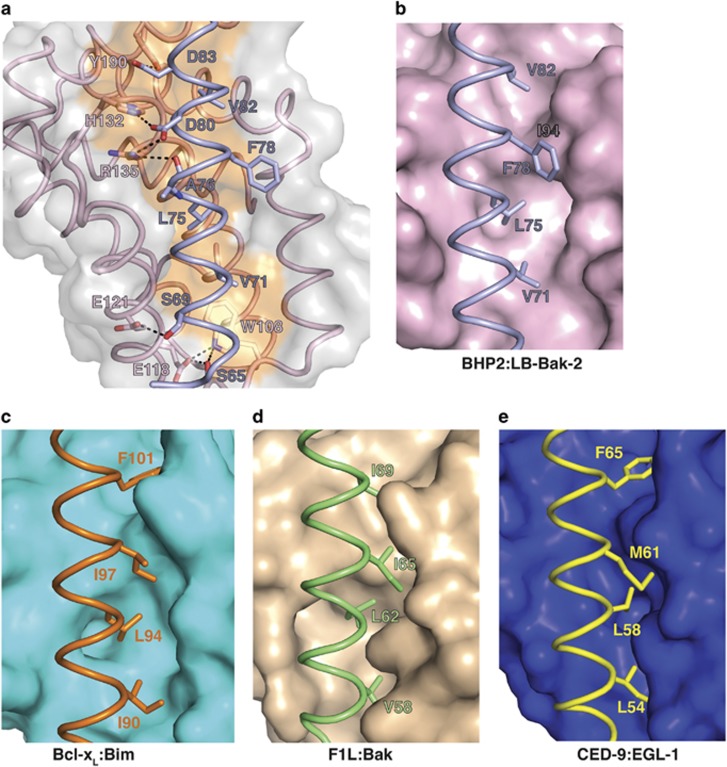
(a) Detailed view of the BHP2:LB-Bak-2 BH3 interface. The BHP2 surface, backbone and binding groove are shown in gray and pink respectively, whereas LB-Bak-2 BH3 is shown in violet. The four key hydrophobic residues of LB-Bak-2 (V71, L75, F78 and V82) on protruding into the binding groove, and the conserved salt bridge formed by LB-Bak D80 and BHP2 R135 are labeled, as well as residues involved in hydrogen bonds. Comparison of the P3 pocket among pro-survival Bcl-2: pro-apoptotic BH3 domain complexes. (b) BHP2:LB-Bak-2 complex. (c) Bcl-xL:Bim complex. (d) CED9:EGL1 complex. (e) F1L:Bak complex