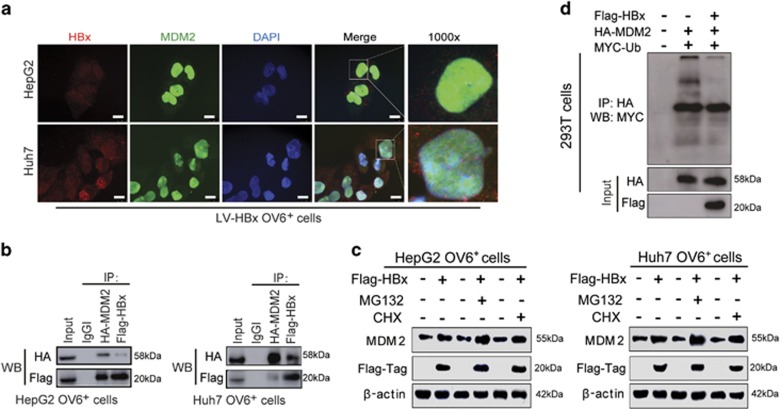
Figure 5.
HBx upregulates the expression of MDM2 by inhibiting its self-ubiquitination and degradation. (a) OV6+ cells were magnetically sorted from HepG2 and Huh7 cells stably expressing HBx, followed by immunofluorescence assays. The localizations of Flag-tagged HBx (red) and MDM2 (green) were detected by confocal laser scanning microscopy. Nucleus were stained by DAPI dye (blue) and representative pictures are shown (scale bar=10 μm). (b) Flag-HBx and HA-MDM2 were cotransfected into HCC (HepG2 and Huh7) cells and OV6+ cells were magnetically sorted from cell pool at 48 h after transfection. Whole-cell lysates were immunoprecipitated with agarose-conjugated anti-HA-tag or anti-Flag-tag antibody, or control immunoglobulin G, and analyzed by western blotting. (c) OV6+ HCC cells (HepG2 and Huh7) infected with either LV-HBx or LV-Con were treated with 1 μg/ml cycloheximide (CHX) or 5 μM MG132 for 12 h before collecting. Then cell lysates were subjected to western blotting. β-actin was used as internal loading control. (d) The ubiquitination of MDM2 was enhanced after HBx transfection for 48 h. Flag-HBx, HA-MDM2 and MYC-ubiquitin were cotransfected into 293T cells. At 48 h after transfection, whole-cell lysates were immunoprecipitated with agarose-conjugated anti-HA-tag antibody, and analyzed by western blotting with anti-HA-tag and anti-MYC-tag antibodies