Table 2. Hydrosilylation of Vinylarenes.
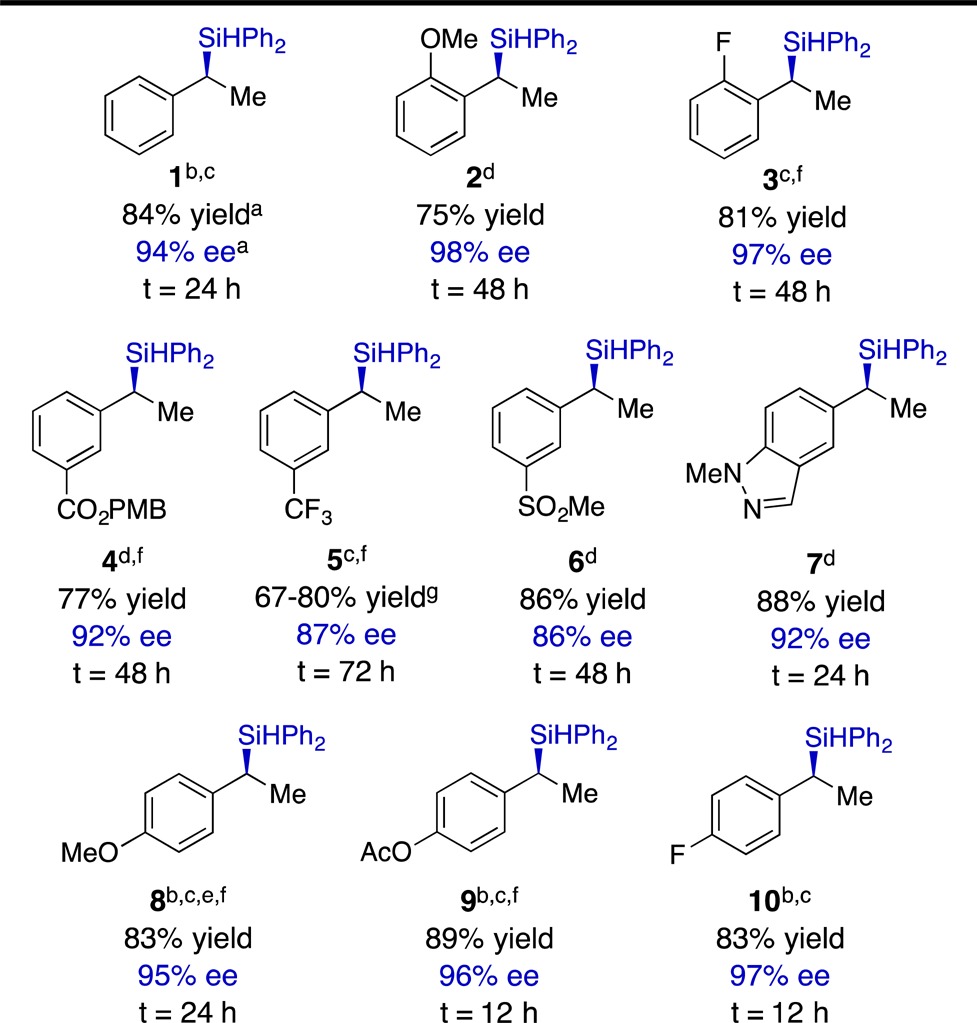
Unless otherwise noted, yields and enantiomeric excesses are the averages for two runs.
Reaction was conducted with 2.0 mol % Cu(OAc)2 and 2.2 mol % (S,S)-Ph-BPE;
Reaction mixture was stirred in a 40 °C oil bath;
Reaction was conducted at ambient temperature;
1.5 equiv. silane were used;
Enantiomeric excesses were determined for the respective silanol derivatives;14
Extrema are the results from two experiments.14
