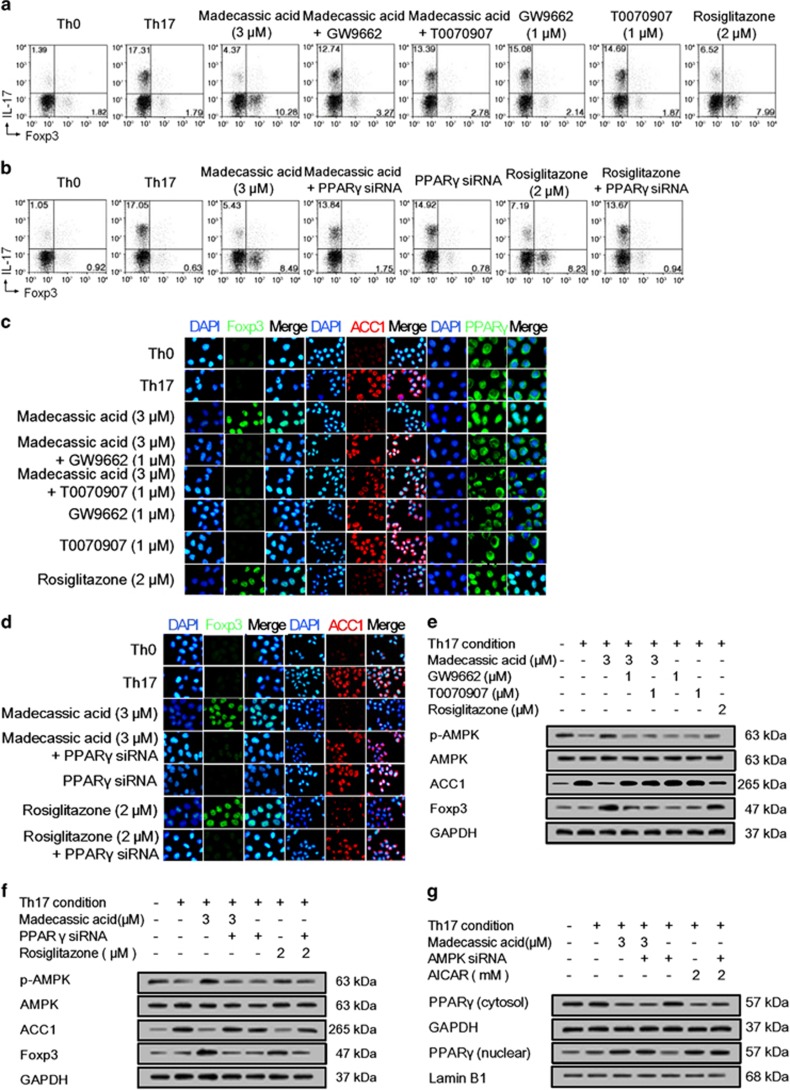
Figure 6.
Madecassic acid regulated AMPK/ACC1 via activating PPARγ in the shift of T helper type 17 (Th17) toward regulatory T cells. Naive T cells were treated with GW9662 (1 μM), T0070907 (1 μM) and PPARγ siRNA under Th17-polarizing conditions for 4 days in the presence of madecassic acid (3 μM). (a) Naive T cells were treated d with GW9662 (1 μM) and T0070907 (1 μM) under Th17-polarizing conditions for 4 days in the presence of madecassic acid (3 μM). The proportions of CD4+IL-17+ T cells and CD4+Foxp3+ T cells were evaluated by flow cytometry. (b) Naive T cells were treated with madecassic acid (3 μM) and PPARγ siRNA under Th17-polarizing conditions for 4 days. The proportions of CD4+IL-17+ T cells and CD4+Foxp3+ T cells were evaluated by flow cytometry. (c) The relative expression of PPARγ, ACC1 and Foxp3 was evaluated by immunofluorescence in the presence of madecassic acid (3 μM), GW9662 (1 μM) and T0070907 (1 μM). (d) The relative expression of ACC1 and Foxp3 was evaluated by immunofluorescence in the presence of madecassic acid (3 μM) and PPARγ siRNA. (e) Naive T cells were treated with GW9662 (1 μM) and T0070907 (1 μM) under Th17-polarizing conditions for 4 days in the presence of madecassic acid (3 μM). The relative expression of p-AMPK, ACC1 and Foxp3 was evaluated by western blot. (f) Naive T cells were treated with madecassic acid (3 μM) and PPARγ siRNA under Th17-polarizing conditions for 4 days. The relative expression of p-AMPK, ACC1 and Foxp3 was evaluated by western blot. (g) Naive T cells were treated with madecassic acid (3 μM) and AMPK siRNA under Th17-polarizing conditions for 4 days. The relative expression of cytosolic and nuclear PPARγ was measured by western blot. GAPDH was used as a cytoplasm marker; Lamin B1 was used as a nuclear marker. All data were expressed as means±S.E.M., n=3. #P<0.05, ##P<0.01 versus Th0 group; *P<0.05, **P<0.01 versus Th17 group; $P<0.05, $$P<0.01 versus madecassic acid group