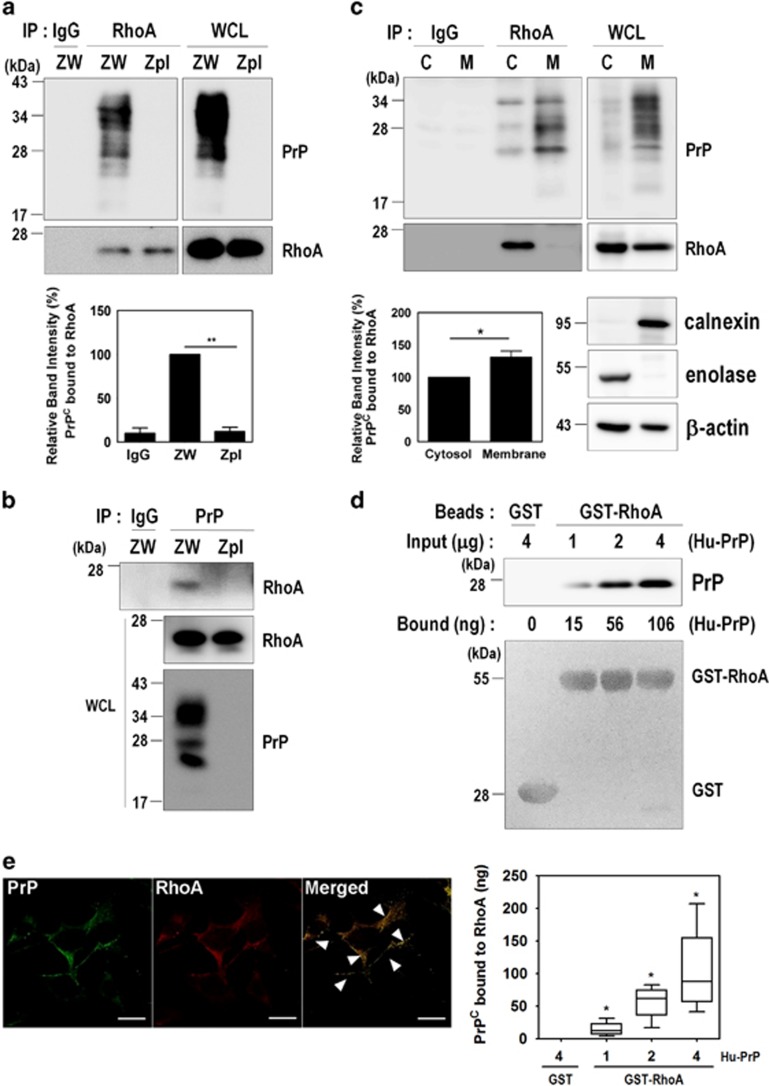
Figure 5.
PrPC interacts with RhoA. (a and b) Co-immunoprecipitation of PrP with RhoA using ZW and Zpl cell lysates were performed with either anti-RhoA (a) or anti-PrP (3F10) (b) antibodies, and then analyzed by western blot with anti-PrP and anti-RhoA antibodies, respectively. WCL, whole-cell lysates. (c) The subcellular fractions from ZW cells were used to immunoprecipitate RhoA with anti-RhoA antibody and then analyzed by western blot with anti-PrP (3F10) and anti-RhoA antibodies. Enolase and calnexin were used as makers for the cytosol (C) and membrane (M) fractions, respectively. β-Actin as a loading control. (d) GST and GST-RhoA beads were incubated with human recombinant PrP (Hu-PrP) as indicated, and the level of Hu-PrP bound to GST-RhoA was determined by western blot with anti-PrP (3F4) antibody. The boxplot showing the means±S.E. of abundance of the PrP-RhoA complex, was calculated from the BSA standard curve in three (n=3) independent experiments. The GST and GST-RhoA samples were stained with Ponceau S to confirm the equal loading. (e) Colocalization of PrP with RhoA was assessed by double immunofluorescence staining and confocal microscopy. All above data are expressed as the mean±S.E. of three independent experiments (*P<0.05, **P<0.01, n=3)