Figure 4.
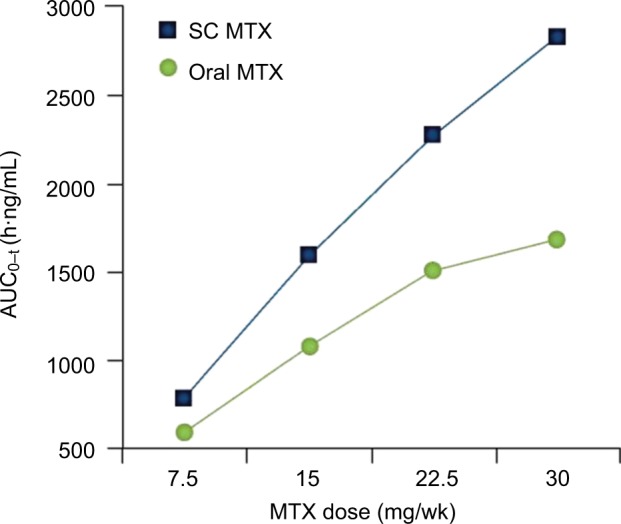
Results from a single-center, open-label, randomized, 2-period, 2-sequence, single-dose, crossover study in 4 dose groups (7.5, 15, 22.5, and 30 mg) with 54 healthy adults treated with oral and subcutaneous MTX.
Note: © 2014 Clinical and Experimental Rheumatology. Reproduced from Pichlmeier U, Heuer KU. Subcutaneous administration of methotrexate with a prefilled autoinjector pen results in a higher relative bioavailability compared with oral administration of methotrexate. Clin Exp Rheumatol. 2014;32(4):563–571.59
Abbreviations: MTX, methotrexate; SC, subcutaneous; AUC, area under the curve.
