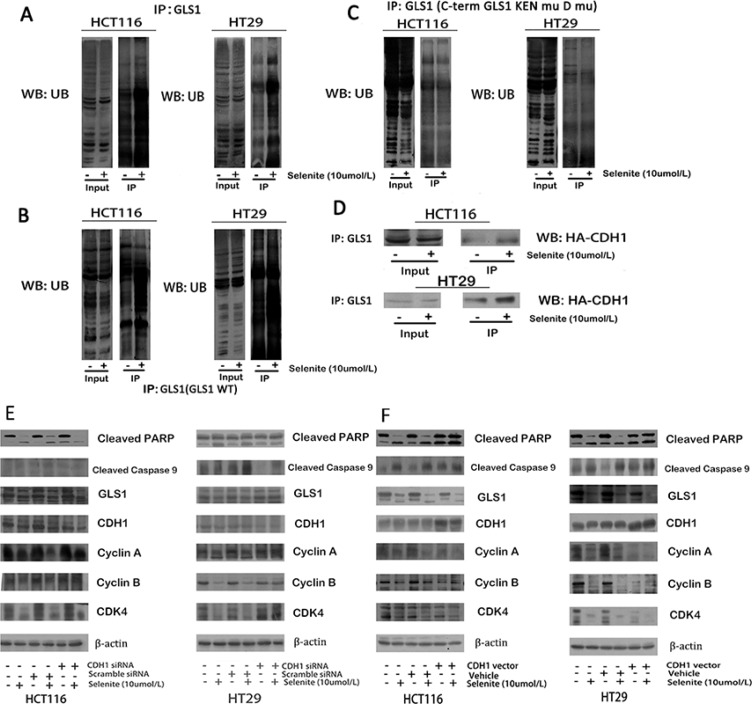
Figure 4. Selenite enhances GLS1 degradation by promoting association between CDH1 and GLS1.
(A) Selenite (10 umol/l) treatment enhanced the interaction between GLS1 and ubiquitin. Ubiquitin was immunoprecipitated from selenite-treated and control cells by GLS1 antibody. The interaction between GLS1 and ubiquitin in the immunopreciptates was analyzed by western blot assay. All the blots were representative of three independent experiments. (B, C) APC/C-CDH1 recruited GLS by recognizing D box and KEN box, a prerequisite in selenite (10 umol/l) enhanced degradation of GLS. Cells were transfected with GLS wild-type or D box and KEN box double mutational vector prior to selenite treatment for 24 h and ubiquitin was immunoprecipitated from selenite-treated and control cells by GLS antibody. (D) Selenite enhanced the interaction between GLS and CDH1. Cells were transfected with HA-CHD1 vector prior to selenite treatment for 24 h and HA was immunoprecipitated from selenite-treated and control cells by GLS1 antibody. Selenite-enhanced PTEN modulated the APC/C-CDH1/GLS1 signaling pathway. (E, F) CDH1 promoted selenite-mediated degradation of GLS. Cells were transfected with CDH1 siRNA or CDH1 plasmids, then cells were treated with or without selenite for 24 h, and western blot was performed to analyze the expression levels of cyclin A, cyclin B, cleaved-PARP, cyclin D, GLS1, cleaved-Caspase. B-actin was used as a loading control.