Abstract
Various minimally invasive techniques have been reported as an alternative to conventional lumbar decompression. The major advantage of these minimally invasive procedures lies in their reduction of unnecessary exposure and tissue trauma. Our objective was to describe a minimally invasive procedure for lumbar spinal stenosis decompression by enlarging the lumbar interspinous space, approaching it with a tubular retractor, and assisting with microscopy. Thoracolumbar fascia and paravertebral muscles are preserved throughout the whole procedure. Iatrogenic instability of the spine can be avoided if during the procedure both joints are just undercut in order to decompress the subarticular space. The approach described in this manuscript could be used as an alternate minimally invasive surgical procedure for the treatment of central and lateral lumbar spinal stenosis.
Keywords: Spinal stenosis, lumbar region, minimally invasive, surgical decompression, spine surgery, minimal access
Introduction
Lumbar spinal stenosis is a disease that has a great social and economic relevance. The pathology affects more than 200,000 people in the United States per year, resulting in substantial pain and disability (1). Lumbar spinal stenosis is a clinical syndrome of buttock or limb pain, which may occur with or without back pain, associated with diminished space available for the neural and vascular elements in the lumbar spine (2). The features used to define lumbar spinal stenosis are: the narrowing of the central spinal canal and nerve root foramina. Whereas, some structural findings in the disease are: hypertrophy of the ligamentum flavum, degenerated facet joints, and bulging intervertebral disc. In 1954, Henk Verbiest introduced the concept of lumbar spinal stenosis as a pathology and established laminectomy as treatment (3). Since then, the development of techniques to decompress the lumbar spinal canal have been published in medical literature and implemented clinically trying to avoid the instability caused by conventional laminectomy (3-5). Conservative management is almost always indicated in patients during the beginning of the disease, even though surgery is generally considered to be the gold standard (6). This paper aims to present another technique based on a microscopy-assisted tubular approach applied in an anatomical corridor between cranial and caudal spinal processes of the lumbar spine, with the objective to decompress the neural elements.
Surgical technique
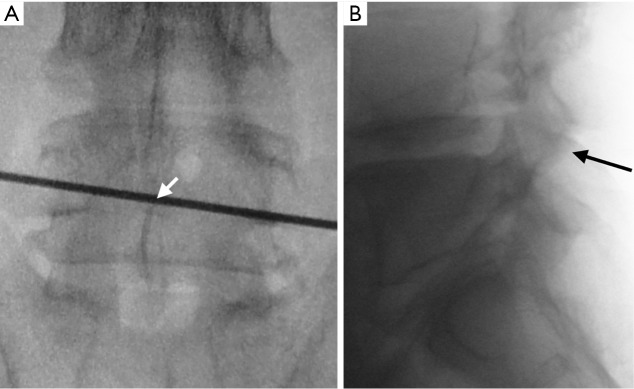
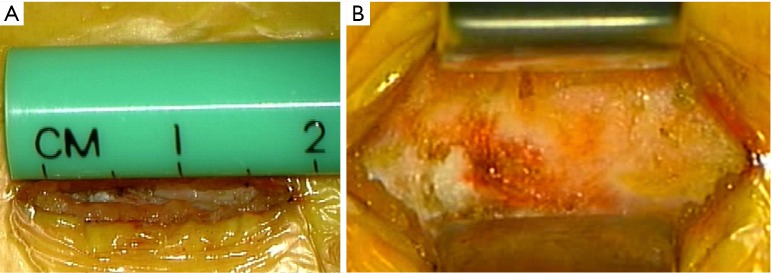
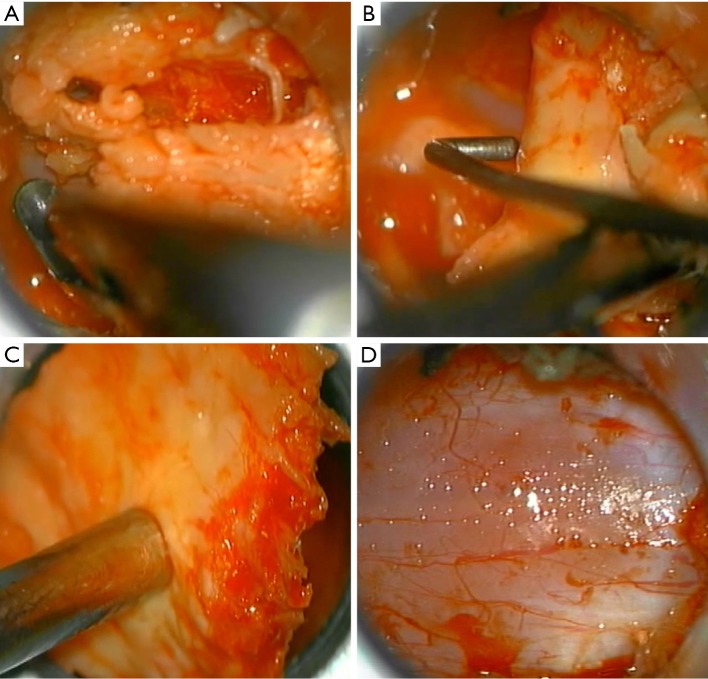
The approach presented can be performed in any patient diagnosed with central or lateral lumbar spinal stenosis, regardless of the number of affected segments. In case of instability, the approach could be accompanied by stabilizing techniques. Some clinical conditions associated with lumbar spinal stenosis that should be taken into account prior to performing the procedure are: mechanical low back pain as the main symptom, instability, and scoliosis with olisthesis. The patient must be positioned in prone over the support frame under general anesthesia. The shoulders should not be abducted more than 90 degrees to avoid a brachial plexus traction. Elbows are flexed at 90 degrees. Intensified fluoroscopy images in antero-posterior and lateral projections are obtained in order to identify the midline and the interspinous level that is to be decompressed (Figure 1). The following steps will be performed under operating microscope. A 2 cm skin incision is made in the midline between the upper and lower spinous processes of the level to be decompressed (Figure 2). The thoracolumbar fascia, supra- and interspinous ligaments are split longitudinally on the midline to avoid total detachment of the ligamentous structures of the spinous processes. A subperiosteal dissection is carried out from the lower third of the cranial spinous process to the upper third of the caudal spinous process. It is important to note that thoracolumbar fascia, supra- and interspinous ligaments are specifically split by the midline and not resected or cut. At the end of decompression, the fascia and ligaments are attached with a suture and reconstructed on the midline. The removal of the lower third of the cranial spinous process and the upper third of the caudal spinous process is done using a high-speed drill (Figure 3). Then, the tubular retractor is placed in the previously enlarged interspinous space (Figure 4). Next, the caudal margin of the upper lamina and the cranial margin of the lower lamina are drilled to complete the entire exposure of the ligamentum flavum. We dissect the yellow ligament using a nerve hook placed in the epidural space. The ligament flavum is then removed completely using a Kerrison rongeur (Figure 5). The bilateral facet joints are undercut medially to expose the lateral margin of the ligamentum flavum and this allows the decompression of the subarticular area, which will also be performed using a Kerrison rongeur or high-speed drill depending on the surgeon’s ability. During this step, the decompression can be facilitated by tilting the tubular retractor to each side in order to visualize the central and lateral canal and thus reach the lateral aspect of the thecal sac, as well as the exiting and traversing roots. Finally, after having achieved an adequate decompression, the wound closure is done in layers. When a multiple level decompression is needed, a 2 cm skin incision is centered over the intermediate spinous process between the top and the bottom spinous processes. Subsequently, the upward and downward retraction of the skin allows the tubular retractor be placed in the upper and lower interspinous space, where the decompression will be made. In case of treating an overweight patient, larger instruments as well as wider and longer tubular retractors can be used.
Figure 1.
Establishing the approach utilizing fluoroscopic imaging. (A) Antero-posterior projection to mark the interspinous level to be decompressed; (B) lateral projection to confirm the interspinous level.
Figure 2.
The incision is performed between both spinous processes. (A) 2 cm skin incision previous to dissecting by planes and performing the tubular approach; (B) thoracolumbar fascia exposed in order to be split.
Figure 3.
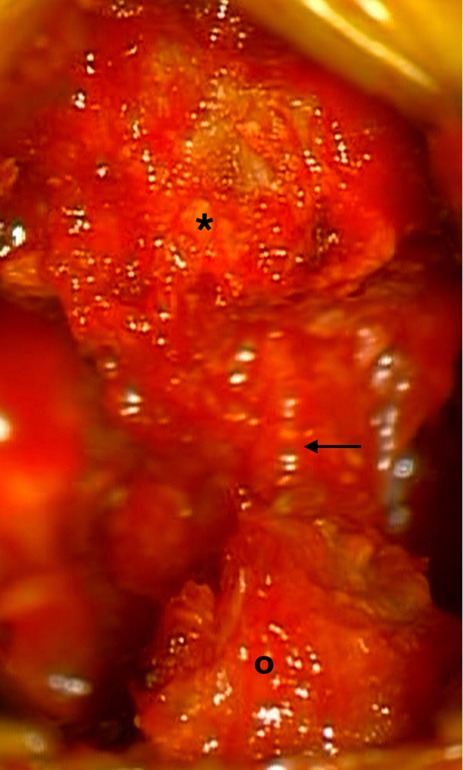
Anatomical image shows the lower third portion of the superior spinous process (*) and the superior third portion of the inferior spinous process (º). Black arrow shows the interspinous space that will be enlarged after drilling both spinous processes.
Figure 4.
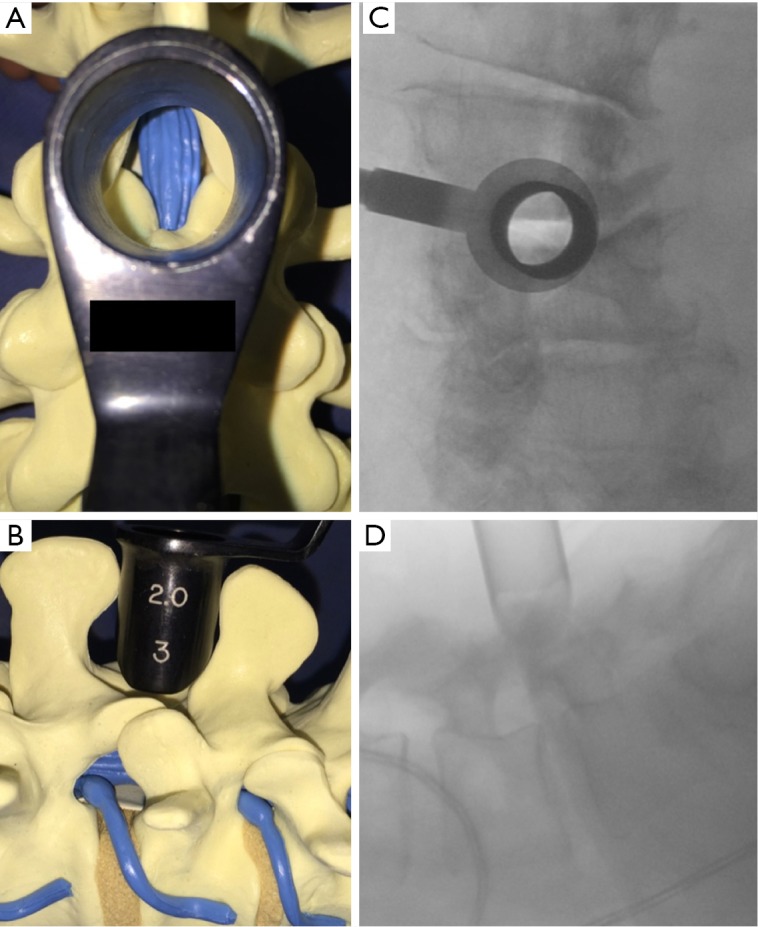
Illustration demonstrating an interspinous space tube placement. (A,B) Schematic diagram illustrates how to place the tube in the interspinous space; (C,D) perioperative fluoroscopic projections with tube placed in the interspinous space.
Figure 5.
Microsurgical trans-operative photograph showing a flavum ligament resection. (A) The superior and inferior insertions of the yellow ligament are freed using a fine Kerrison rongeur; (B) yellow ligament is dissected using a nerve hook; (C) yellow ligament is extracted; (D) lumbar spinal segment is decompressed with dura mater exposed.
Demonstrative clinical case
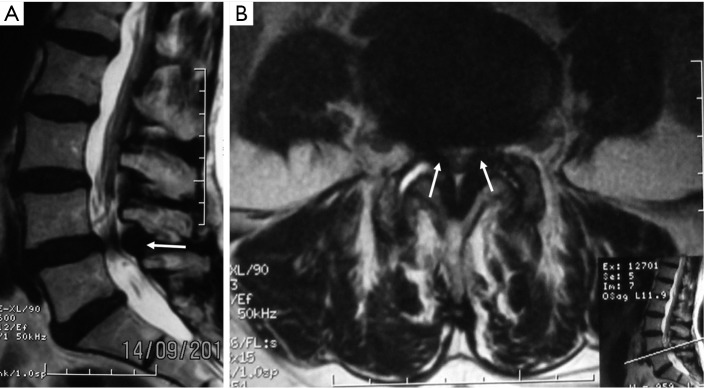
A 75-year-old man presented with progressive weakness and radicular pain of both legs with 2 months of evolution. The physical examination revealed bilateral weakness of both knees. The radiological protocol using magnetic resonance imaging (MRI) demonstrated lumbar spinal stenosis in L4–L5 level (Figure 6). An interspinous tubular approach was done in L4–L5 level (Figure 7). The patient improved his symptoms and was discharged from hospital the next day without any complications.
Figure 6.
Preoperative magnetic resonance image (MRI) showing the lumbar spinal canal stenosis in L4–L5. (A) T2-weigthed sagittal view; (B) T2-weigthed axial view. White arrows show hypertrophy of the yellow ligament.
Figure 7.
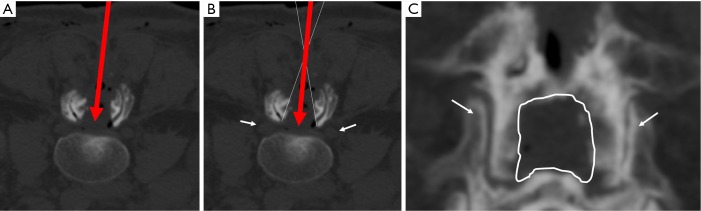
Postoperative lumbar computed tomography (CT) imaging. (A) CT-axial view showing the surgical corridor with the interspinous approach (red arrow); (B) same CT-axial view showing the extension of the decompression and how the tube can be moved in order to reach the subarticular area (white lines); (C) 3D-CT reconstruction in posterior-anterior view. The interlaminar enlarged space and both facet joints (white arrows) are preserved as shown.
Discussion
The approach presented in this manuscript implements the tubular retractor to the procedure described as a muscle-preserving interlaminar decompression (MILD) in order to make it more comfortable, faster, and safer. It also reduces injury to the skin and thoracolumbar fascia and supra- and interspinous ligaments. It also decreases the retraction over paraspinal muscles (7). The microscopic assistance used throughout the whole procedure is very important in order to carry out a fine dissection between ligamentum flavum and spinal dura. Tubular approaches applied to the spine have proved to be safer with less postoperative wound pain, shorter postoperative recovery time, and better clinical outcomes (8,9). Some of the most important advantages of minimally invasive lumbar decompression relate to the ability to perform adequate neural element decompression while preserving the facet joints and back muscles, avoiding instability in the lumbar spine (10). Biomechanical studies have proven that the elements of the facet, including the capsule, should be preserved as much as possible during the decompression procedure. Other studies suggested that medial facetectomy does not affect lumbar spinal stability. Conversely, total facetectomy even when performed unilaterally, creates instability in the lumbar spine (11). Another important anatomical structure preserved in the procedure is the thoracolumbar fascia. This fascia serves as an important dynamic stabilizer for the lumbar spine, and a connection between the spinous processes and the muscles such as the latissimus dorsi, transverses abdominis, and erector spinae (12,13). There are several advantages that we can evaluate on this approach: (I) the tubular retractor application in the interspinous space is technically easier than Gelpi self-retaining system or Caspar retractor system because the latter two are void of a support that mounts to the surgical table; (II) the skin incision necessary for this approach is only 2 cm even when performing a multilevel decompression; (III) the interspinous tubular approach is faster because it requires less dissection of the supra- and interspinous ligaments; (IV) the lateral and cranio-caudal decompression can be facilitated by redirecting the tubular retractor in order to reach the superior, inferior, and lateral attachments of the yellow ligament; (V) the technique lends itself to a faster learning curve than other more complex minimally invasive surgery (MIS) techniques; (VI) the ligamentum flavum can be removed while preserving the facet joints during the decompression, obviating the need for subsequent fusion to treat iatrogenic instability; (VII) the microsurgical-volumetric increase of the central spinal canal as well as the lateral recesses with a small tubular retractor can be accomplished while preserving the posterior tension band and the majority of the lumbar spinous processes; (VIII) early out of bed mobilization of the patient, minimal postoperative pain, less use of postoperative narcotics, and decreased incidence of complications such as deep venous thrombosis (DVT), urinary tract infections, and pneumonia.
Conclusions
This approach is an effective procedure used to treat lumbar spinal stenosis and it has the benefit of being performed with minimal access of the tubes. This translates into a shorter time to prepare the lumbar interspinous corridor in order to achieve the interlaminar window. The approach described in this manuscript could be used as another minimally invasive surgical procedure for the treatment of lumbar spinal stenosis.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 2010;303:1259-65. 10.1001/jama.2010.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watters WC, 3rd, Baisden J, Gilbert TJ, et al. Degenerative lumbar spinal stenosis: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J 2008;8:305-10. 10.1016/j.spinee.2007.10.033 [DOI] [PubMed] [Google Scholar]
- 3.Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br 1954;36-B:230-7. [DOI] [PubMed] [Google Scholar]
- 4.Johnsson KE, Willner S, Johnsson K. Postoperative instability after decompression for lumbar spinal stenosis. Spine (Phila Pa 1976) 1986;11:107-10. 10.1097/00007632-198603000-00001 [DOI] [PubMed] [Google Scholar]
- 5.Lee CK. Lumbar spinal instability (olisthesis) after extensive posterior spinal decompression. Spine (Phila Pa 1976) 1983;8:429-33. 10.1097/00007632-198305000-00014 [DOI] [PubMed] [Google Scholar]
- 6.Negrini S, Zaina F, Romano M, et al. Rehabilitation of lumbar spine disorders: an evidence-based clinical practice approach. In: Frontera WR. editor. DeLisa's Physical Medicine and Rehabilitation: Principles and Practice. 5th Edition. Lippincott Williams & Wilkins, 2010:837-82. [Google Scholar]
- 7.Hatta Y, Shiraishi T, Sakamoto A, et al. Muscle-preserving interlaminar decompression for the lumbar spine: a minimally invasive new procedure for lumbar spinal canal stenosis. Spine (Phila Pa 1976) 2009;34:E276-80. 10.1097/BRS.0b013e318195d943 [DOI] [PubMed] [Google Scholar]
- 8.Pao JL, Chen WC, Chen PQ. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur Spine J 2009;18:672-8. 10.1007/s00586-009-0903-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu RH, Fraser JF, Härtl R. Minimal access versus open transforaminal lumbar interbody fusion: meta-analysis of fusion rates. Spine (Phila Pa 1976) 2010;35:2273-81. 10.1097/BRS.0b013e3181cd42cc [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa K, Kitahara K, Shimoda H, Hara T. Biomechanical evaluation of destabilization following minimally invasive decompression for lumbar spinal canal stenosis. J Neurosurg Spine 2013;18:504-10. 10.3171/2013.1.SPINE12599 [DOI] [PubMed] [Google Scholar]
- 11.Abumi K, Panjabi MM, Kramer KM, et al. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila Pa 1976) 1990;15:1142-7. 10.1097/00007632-199011010-00011 [DOI] [PubMed] [Google Scholar]
- 12.Bogduk N, Johnson G, Spalding D. The morphology and biomechanics of latissimus dorsi. Clin Biomech (Bristol, Avon) 1998;13:377-85. 10.1016/S0268-0033(98)00102-8 [DOI] [PubMed] [Google Scholar]
- 13.Bogduk N, Macintosh JE. The applied anatomy of the thoracolumbar fascia. Spine (Phila Pa 1976) 1984;9:164-70. 10.1097/00007632-198403000-00006 [DOI] [PubMed] [Google Scholar]