Abstract
The aim of this study is to compare the perioperative outcomes between laparoscopic and open resections performed for colonic emergencies. A systematic search of the literature identified previously published comparative studies regarding emergent laparoscopic colectomy (ELC) and emergent open colectomy (EOC). Meta-analysis was performed utilizing a pooled odds ratio (OR) for dichotomous variables and a weighted mean difference (WMD) for continuous variables with 95 % confidence intervals (CIs). Eleven studies involving 752 patients were identified. Although operation time was noted to be significantly shorter for EOC, patients post-ELC had significantly lower overall morbidity (OR 0.44; 95 % CI 0.30, 0.66; P < 0.0001). Meanwhile, recovery time for post-ELC patients was significantly shorter, as was the length of hospital stay (WMD −2.78 days; 95 % CI −3.17, −2.38; P < 0.00001), the time to regular dietary habits (WMD −1.32 days; 95 % CI −2.51, −0.13; P = 0.03), and the time to recover bowel movement (WMD −0.55 days; 95 % CI −0.89, −0.22; P = 0.001). Reoperation rate and mortality were found to be comparable between ELC and EOC. The R0 resection rate and the number of lymph nodes harvested were also comparable between ELC and EOC for malignant diseases. Whether for benign or malignant disease, ELC is a safe and feasible procedure for colonic emergencies compared with EOC, despite being relatively time-consuming.
Keywords: Laparoscopic colectomy, Minimally invasive surgical procedures, Colectomy, Emergencies, Meta-analysis
Introduction
The introduction of abdominal laparoscopic surgery revolutionized the field of general surgery over the past decades, with the former’s advantages over open surgery well established, particularly for elective patients with benign or malignant pathology. As well-trained minimally invasive specialty surgeons increased in number, laparoscopic surgery became a feasible and effective alternative to laparotomy for several diseases in the emergency setting as well. The best indications for laparoscopy in case of an emergency include acute appendicitis, calculous cholecystitis, and a perforated peptic ulcer [1].
Due to the shorter length of hospital stay (LOS), quicker recovery, and lower postoperative morbidity, laparoscopic colorectal resection was commonly preferred to open procedures in elective conditions [2]. Thus, the technical demand of laparoscopy for emergent colectomy became a matter of course. Although over one third of urgent surgical admissions were for colorectal pathology [3], the role of laparoscopic colectomy (LC) in the emergency setting remains controversial. The major drawback of emergent laparoscopic colectomy (ELC) was lack of encouraging highly reliable evidence, with a particular weakness in prospective randomized control trials (RCTs). Additionally, most of the aforementioned studies were performed in single-center settings and were retrospective in design. Furthermore, some research even produced negative conclusions: a recent study indicated that the emergent laparoscopic Hartmann procedure for complicated diverticulitis could decrease neither the postoperative morbidity nor the mortality when compared with emergent open colectomy (EOC) [4].
This analysis was conducted in order to break a deadlock set in place due to recent conflicts of evidence. Both randomized and nonrandomized studies were considered, since the reliability of high-quality nonrandomized comparative studies was proven to be as powerful as RCTs when adopted for a meta-analysis [5]. We established no restrictions concerning pathological diagnoses since urgent resection of colorectal malignancies is also becoming a major part of routine emergency department work.
Materials and Methods
Search Strategy
An electronic bibliographic search was carried out using the following databases: MEDLINE (PubMed), Embase, and The Cochrane Library. The following search headings were utilized to identify all studies published up to July 2015 in English: “emergency,” “laparoscopic,” and “colectomy.” Related articles additionally broadened results, and all abstracts, studies, and citations scanned were reviewed.
Inclusion Criteria
Included studies satisfied all of the following criteria: (1) the publication must have described a comparative study; (2) the study had to compare the characteristics and perioperative outcomes of adult patients undergoing laparoscopic and open colectomy (OC); (3) all operations had to be performed in an emergency setting; and (4) the disease had to be comparable between ELC and EOC.
Exclusion Criteria
Studies were excluded from the analysis when the outcomes and parameters of patients were not reported or deemed impossible to calculate for both groups. Single-port laparoscopic and robot-assisted laparoscopic operations were not included in the analysis. Studies were also excluded when the two groups dealt with different diseases or the diagnosis of disease was not reported. If cases were duplicately published, only the most informative and recent study was included.
Data Extraction and Quality Assessment
Two reviewers independently extracted the following parameters from each study: first author(s), year of publication, population characteristics, study design, numbers of subjects undergoing each procedure, and perioperative outcomes. Disagreements were resolved by discussion.
The Newcastle Ottawa scale (NOS) was adopted to assess the quality of nonrandomized studies. Three parameters of studies were evaluated to determine adequate quality: representation of the patient population, comparability of two patient groups, and reliability of outcomes. A study, at most, had nine stars as a full score. Studies with a score equal to or higher than seven were considered methodologically sound.
Outcomes of Interest
The following relevant data were extracted from the studies: population characteristics, intraoperative outcomes (operative time and intraoperative blood loss), postoperative recovery (LOS and time to resumption of normal diet), postoperative complications (overall morbidity, intra-abdominal abscess, intra-abdominal bleeding, and prolonged ileus), reoperation rate, postoperative mortality, and oncological outcomes (R0 resection rate and lymph nodes harvested).
Subgroup Analyses
To further analyze the effects of pathology on postoperative outcomes, subgroup analyses were carried out according to pathologic diagnosis.
Statistical Analysis
This meta-analysis was conducted according to guidelines of Meta-analysis of Observational Studies in Epidemiology (MOOSE) [6]. We analyzed dichotomous variables using estimation of odds ratio (OR) and continuous variables using weighted mean difference (WMD) with a 95 % confidence interval (CI). If the study provided medians and ranges instead of means and standard deviations (SD), we estimated the means and SDs as described by Hozo et al. [7]. P < 0.05 indicated a statistically significant difference between the two groups. Before performing meta-analysis, homogeneity of effect sizes was assessed by χ 2 and I 2. We considered heterogeneity to be present if the I 2 statistic was >50 %. A fixed-effect (Mantel–Haenszel) statistical model was used in the absence of significant heterogeneity; otherwise, a random effects (DerSimonian and Laird) model was utilized. Publication bias was examined by the symmetry of funnel plot. Statistical analyses were performed using Review Manager (RevMan) version 5.1.0.
Results
Results of Study Search and Quality Assessment
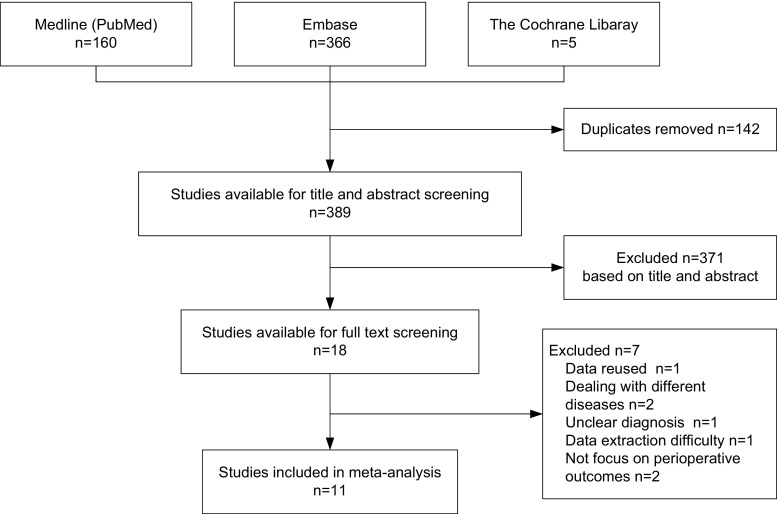
The bibliographic search identified 389 potentially relevant studies, and subsequent title and abstract screening eliminated 371 studies that obviously did not fit our inclusion criteria. Out of the remaining 18 studies, 11 studies [4, 8–17] were finally included in our meta-analysis after a full-text review (Fig. 1).
Fig. 1.
Flowchart of studies retrieved from literature search
There was no RCT component in this analysis. All 11 studies included were retrospective and nonrandomized in design, 5 studies were cohort, and 6 were case-matched studies. Results of quality assessment were satisfactory; three studies got seven stars, six studies got eight stars, and the remaining two got nine stars. No included study was evaluated as being low quality. All dates of surgery mentioned in studies were after 2000, except for those reported by Dunker et al. [9] and Marcello et al. [14]. The sample size of these studies ranged from 18 to 134. Every study recorded at least two of three baseline parameters, containing age, gender, and body mass index. All studies were comparable with age and gender, except for two studies [11, 17]. The basic characteristics and baseline data of included studies are listed in Table 1.
Table 1.
The characteristics and baseline parameters of the included studies
| Author | Year | Country | Design | Laparoscopic approach | Pathology | No. of patients | Age | Gender (male) | BMI | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ELC | EOC | ELC | EOC | ELC | EOC | ELC | EOC | Score | ||||||
| Catani M | 2011 | Italy | Case-match | Lap | Ben, Mal | 32 | 61 | 50.1 ± 19.15 | 54.9 ± 16.38 | 19 | 29 | NR | NR | 8 |
| Dunker MS | 2000 | The Netherlands | Cohort | Lap, HALS | Ben | 10 | 32 | 33 ± 7.7 | 37 ± 12.1 | 3 | 14 | 19.7 ± 4.1 | 20.9 ± 3.5 | 7 |
| Koh FH | 2013 | Singapore | Case-match | Lap, HALS | Ben, Mal | 23 | 23 | NA | NA | 13 | 13 | NR | NR | 8 |
| Letarte F | 2014 | Canada | Cohort | Lap | Ben | 39 | 86 | 61.6 ± 13.7 | 60.9 ± 12.0 | 12* | 53* | 26.3 ± 4.0 | 26.7 ± 4.5 | 8 |
| Li JC | 2009 | Hong Kong, China | Cohort | Lap | Ben | 6 | 12 | 47 (30–87) | 48.5 (16–68) | 4 | 6 | NR | NR | 7 |
| Li Z | 2015 | China | Case-match | HALS | Mal | 10 | 25 | 64.5 ± 9.7 | 62.3 ± 10.2 | 6 | 14 | 22.2 ± 3.4 | 22.3 ± 4.1 | 9 |
| Marcello PW | 2001 | USA | Case-match | Lap | Ben | 19 | 29 | 32 (15–60) | 33 (20–58) | 10 | 13 | 24 (17–34) | 24 (16–36) | 8 |
| Ng SS | 2008 | Hong Kong, China | Cohort | Lap | Mal | 14 | 29 | 68.5 (45–80) | 71 (44–94) | 6 | 14 | 21.1 (18.6–35.5) | 20.7 (17.8–26.9) | 8 |
| Odermatt M | 2013 | UK | Case-match | Lap | Mal | 36 | 72 | 74 (32–93) | 77.5 (30–92) | 15 | 36 | 24 (16–41) | 26 (15–38) | 8 |
| Turley RS | 2013 | USA | Case-match | Lap | Ben | 67 | 67 | 58.5 ± 16.3 | 59 ± 13.5 | 41 | 40 | 27.6 ± 7.8 | 26.7 ± 6.5 | 9 |
| Watanabe K | 2009 | Japan | Cohort | HALS | Ben | 30 | 30 | 26.9 (13–64)* | 35.7 (19–84)* | 17 | 19 | 18.6 (11.5–24.2) | 18.5 (13.8–22.5) | 7 |
NA not assessable, NR not reported, Lap laparoscopy, HALS hand-assisted laparoscopic surgery, Ben benign, Mal malignant, BMI body mass index
*P < 0.05
Intraoperative Outcomes
Eight studies [4, 8, 10–14, 17] reported operative time. Pooled data of these eight studies indicated that an open procedure was more time-efficient (random effects model; WMD 36.47 min; 95 % CI 12.07, 60.87; P = 0.003). Six studies [11–15, 17] reported the volume of intraoperative blood loss. Pooled data of the six studies revealed that patients under ELC lost comparable blood as whom under EOC (random effects model; WMD −54.00 ml; 95 % CI −129.66, 21.65; P = 0.16).
Outcomes of Morbidity and Mortality
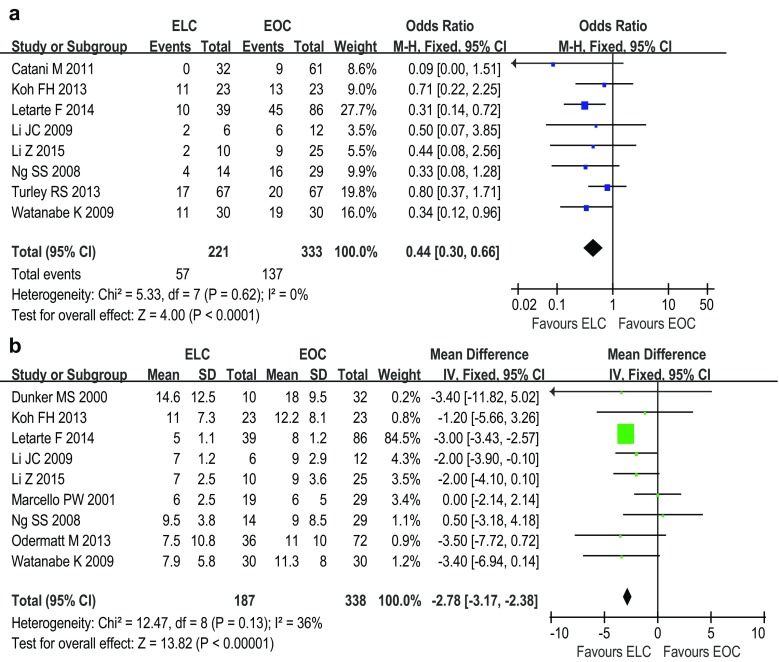
Eight studies [4, 8, 10–13, 15, 17] respectively indicated that the morbidity in the ELC group was lower than that in the EOC group. Pooled data of these ten studies confirmed that patients after ELC were significantly more likely to avoid postoperative complications than whom after EOC (fixed effects model; OR 0.44; 95 % CI 0.30, 0.66; P < 0.0001) (Fig. 2a). However, the incidences of surgical complications, including wound infection (random effects model; OR 0.63; 95 % CI 0.23, 1.70; P = 0.36), intra-abdominal bleeding (fixed effects model; OR 1.20; 95 % CI 0.30, 4.75; P = 0.79), abdominal abscess (fixed effects model; OR 0.72; 95 % CI 0.28, 1.84; P = 0.49) and prolonged ileus (fixed effects model; OR 0.62; 95 % CI 0.34, 1.13; P = 0.12) were not significantly different between the ELC and EOC groups. Meanwhile, the incidence of reoperation was comparable between ELC and EOC (fixed effects model; OR 0.95; 95 % CI 0.44, 2.04; P = 0.89). Nine studies [4, 8, 10–13, 15–17] reported postoperative mortality. Pooled data of these studies revealed that the incidence of postoperative death was comparable between ELC and EOC (fixed effects model; OR 0.54; 95 % CI 0.23, 1.28; P = 0.16).
Fig. 2.
Forest plots of the meta-analysis of overall postoperative morbidity (a) and the length of hospital stay (b). ELC emergent laparoscopic colectomy, EOC emergent open colectomy
Recovery Outcomes
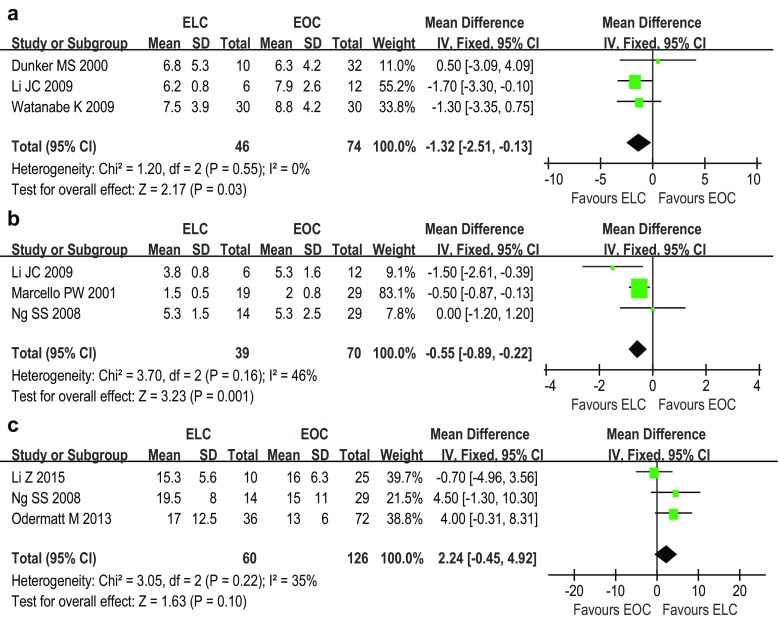
Nine studies [9–17] reported the results of LOS. Pooled data showed that the LOS was significantly shorter for ELC than EOC (fixed effects model; WMD −2.78 days; 95 % CI −3.17, −2.38; P < 0.00001) (Fig. 2b). Three studies [9, 12, 17] reported the time to resume normal diet. Patients following ELC were noted to require significantly less time to resume a normal diet (fixed effects model; WMD −1.32 days; 95 % CI −2.51, −0.13; P = 0.03) (Fig. 3a). Meanwhile, three studies [12, 14, 15] reported the first day to recover bowel movement. Pooled data showed that patients after ELC recovered bowel movement significantly more quickly than whom after EOC (fixed effects model; WMD −0.55 days; 95 % CI −0.89, −0.22; P = 0.001) (Fig. 3b).
Fig. 3.
Forest plots of the meta-analysis of the time to regular dietary habits (a), the time to recover bowel movement (b), and the number of lymph nodes harvested (c). ELC emergent laparoscopic colectomy, EOC emergent open colectomy
Subgroup Analyses
In our included studies, there were six studies [4, 9, 11, 12, 14, 17] dealing with benign diseases. Results of this subgroup analysis kept highly consistent with the results of the aforementioned meta-analysis, including operative duration, intraoperative blood loss, overall postoperative morbidity, wound infection, intra-abdominal bleeding, intra-abdominal abscess, prolonged ileus, reoperation rate, LOS, time to resume diet, and mortality.
Three studies [13, 15, 16] were included into the malignant disease subgroup. Subgroup analyses were performed for three parameters, including wound infection, LOS, and mortality, whose results were also consistent with the results of the aforementioned meta-analysis. The oncological outcomes of malignant disease were also reported by these three studies. Ng et al. [15] did not reported outcomes of R0 resection rate. All patients got R0 resection in Li’s study [13]. The R0 resection rate was comparable in Odermatt’s study [16], 72.2 % for ELC and 88.9 % for EOC, respectively. The number of lymph nodes harvested was similar between ELC and EOC (fixed effects model; WMD 2.24; 95 % CI −0.45, 4.92; P = 0.10) (Fig. 3c).
Outcomes of Heterogeneity
Statistical heterogeneity was observed in three parameters including operative time, wound infection, and blood loss. However, in the relatively more important parameters, there was no statistical heterogeneity, such as LOS, mortality, morbidity, and reopreation rate.
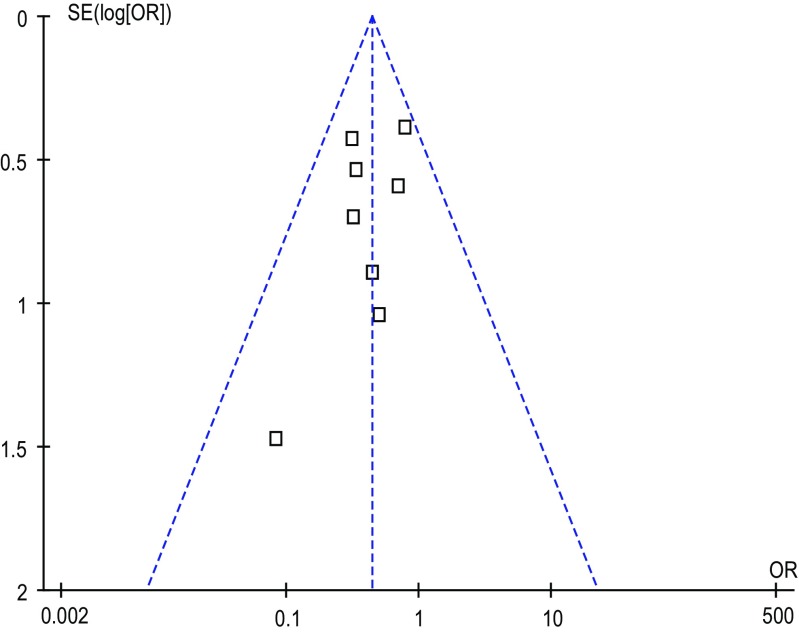
Outcomes of Publication Bias
Funnel plots were constructed for each outcome and showed symmetry, suggesting that publication bias was not large and was unlikely to drive conclusions. The funnel plot of overall morbidity is shown in Fig. 4.
Fig. 4.
Funnel plot of overall postoperative morbidity
Discussion
The benefits of elective LC have been widely confirmed, especially for benign disease [18]. Our analysis confirmed that the benefits of laparoscopy still existed under the emergency setting. This analysis also took malignant disease into consideration, because up to 20 % of colorectal cancers presented as surgical emergencies [19]. Furthermore, these patients who received operative intervention in a timely manner after emergency admission had better outcomes: Gunnarsson et al. [20] reported that colonic cancer patients who underwent colectomy within 3 days after emergency admission had a lower postoperative mortality and a higher 5-year survival rate than those who were operated upon after more than 3 days.
Nowadays, laparoscopy is widely adopted in colonic cancer surgery. Data from eight institutions of the National Comprehensive Cancer Center Network indicated that the proportion of laparoscopy-assisted surgery in colonic cancers was over 50 % in 2010 and the number was deemed increasing as the time passed [21]. An obvious superiority of LC over open resection for colon cancer was supported on the basis of lower postoperative mortality and quicker recovery [22]. Moreover, long-term survival for malignant disease was reported to be comparable between LC and OC [23]. However, there were few studies reporting the oncological safety of ELC for colonic malignancies. To our best knowledge, this is the first meta-analysis which revealed the oncological outcomes of malignant diseases (R0 resection rate and lymph nodes harvested) between ELC and EOC.
Most crucially, malignant pathology has not been a technical barrier to the application of laparoscopy for surgical treatment of colonic disease: Vaccaro et al. [24] stated that independent risk factors for conversion of laparoscopic colorectal resections contained lesion location, body size, and gender, but not disease type, and neither neoplastic or nonneoplasic characteristics. Based on the above realizations, emergent malignant diseases should have been taken into account along with benign surgical diagnoses, which was reinforced by the absence of heterogeneity within major parameters and the stable outcomes of subgroup analyses.
As for important intraoperative parameters, the operative duration of ELC was noted to be significantly longer than that of EOC, according to pooled data.
Firstly, the additional processing time of laparoscopy was probably due to a steep learning curve. A systematic review [25] of 4852 patients demonstrated that the length of the learning curve for laparoscopic colorectal resection ranged from 87 to 152 cases. Concretely, the accumulation of about 100 cases’ worth of experience was required for a stable operative time, and about 150 cases for procedure conversion. Factoring in off-duty surgeries, a lack of sufficient, timely preoperative readiness, and the wide variety of surgical settings surveyed, ELC outcome was undoubtedly influenced by a greater number of unforeseen variables than was elective LC. However, in most of the included studies, the total case quantity was noted to be fewer than 40, and cases were not all from a single surgeon. The heterogeneity of operative duration also reflected a disparity in surgical skills and technical proficiency among surgeons.
Secondly, converted cases were commonly counted into the laparoscopic patient group based on a presumed initial laparoscopic approach. Conversion doubtlessly extended the average operative time by a great deal.
Despite the inferiority regarding time efficiency, ELC was found to conserve as much blood as EOC. Up to 200–250 ml of blood loss was demonstrated to correlate with postoperative complications such as surgical site infection, bowel anastomosis leak, and lowered long-term survival rates [26]. Concurrently, the superiority of LC in regards to morbidity and mortality would disappear when the surgical duration surpassed 3 h [27]. While it is still unknown whether blood conservation or decreased surgery duration alone has a greatest influence on ELC.
Even if each procedure had its own merits and drawbacks among intraoperative outcomes, ELC presented a distinct advantage in overall morbidity. However, meta-analysis revealed ELC to have no clear advantage in events of wound infection, intra-abdominal bleeding, abscess formation, or prolonged ileus, all classified under surgery-related complications. The following reasons may adequately explain such inconsistency: firstly, other surgery-related complications (such as intestinal fistula and anastomotic leakage) resulted in an increased number of operative complications in the EOC group. Previous research [28] demonstrated that 3.6 % of serious complications needing reoperative after colonic resection were intestinal fistula or anastomotic leakage, but only 0.5 % were intra-abdominal bleeding. Unfortunately, such important parameters could not be extracted from included studies.
Post-ELC patients were released from the hospital much earlier, which owes to quicker recovery from laparoscopy. The time to normal diet resumption was about 1 day shorter in the ELC group than that in the EOC group. The lower post-ELC morbidity was associated with a shorter LOS, because postoperative morbidity is an independent risk factor influencing the LOS [29].
In addition to clinical outcomes, economic worth has been perceived to be a limiting factor in the development and popularization of laparoscopic colonic surgery. Under elective conditions, due to application of disposable instruments, the operative cost of LC was higher than that of OC. However, the total in-hospital charge of LC was comparable with OC, sometimes even lower, as was the community cost, due to the lower postoperative morbidity and the shorter LOS [30]. There are few studies on the cost of ELC, but the technical superiority of ELC is likely to evolve into cost-effectiveness as elective LC has. In fact, one [10] of the included studies reported that an in-hospital charge was comparable between ELC and EOC. We expect more studies on the economic comparisons between ELC and OLC in the future.
Limitation of This Meta-Analysis
None of the included studies was of randomized, prospective design, which made the conclusions almost impossible to avoid a selection bias. However, the availability of appropriate surgeons and instruments would be much more uncertain in emergency settings, as well as conditions of patients. Thus, performance of a RCT concerning an emergency setting would also be much more difficult. Fortunately, all included studies were assessed to be of high reliability in nature.
The sample size of each included study was distributed among a wide range. However, the samples of the 11 included studies were considered to be from a similar statistical population, based on heterogeneity factors. Meanwhile, key clinical outcomes kept stable after subgroup analyses.
Conclusions
Whether for benign or malignant disease, ELC is a safe and feasible procedure for colonic emergencies compared with open surgery, despite being relatively time-consuming.
Acknowledgments
Author Contributions
Xu SB: acquisition of data, analysis and interpretation of data, drafting the article, and final approval. Jia Z: acquisition of data, analysis and interpretation of data, drafting the article, and final approval. Zhu YP: interpretation of data, revising the article, and final approval. Zhang RC: interpretation of data, revising the article, and final approval. Wang P: conception and design of the study, critical revision, and final approval.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest concerning this article.
References
- 1.Navez B, Navez J. Laparoscopy in the acute abdomen. Best Pract Res Clin Gastroenterol. 2014;28(1):3–17. doi: 10.1016/j.bpg.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Rondelli F, Trastulli S, Avenia N, Schillaci G, Cirocchi R, Gulla N, Mariani E, Bistoni G, Noya G. Is laparoscopic right colectomy more effective than open resection? A meta-analysis of randomized and nonrandomized studies. Color Dis. 2012;14(8):e447–e469. doi: 10.1111/j.1463-1318.2012.03054.x. [DOI] [PubMed] [Google Scholar]
- 3.Al-Ayoubi F, Eriksson H, Myrelid P, Wallon C, Andersson P. Distribution of emergency operations and trauma in a Swedish hospital: need for reorganisation of acute surgical care? Scand J Trauma Resusc Emerg Med. 2012;20:66. doi: 10.1186/1757-7241-20-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turley RS, Barbas AS, Lidsky ME, Mantyh CR, Migaly J, Scarborough JE. Laparoscopic versus open Hartmann procedure for the emergency treatment of diverticulitis: a propensity-matched analysis. Dis Colon Rectum. 2013;56(1):72–82. doi: 10.1097/DCR.0b013e3182749cf5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abraham NS, Byrne CJ, Young JM, Solomon MJ. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol. 2010;63(3):238–245. doi: 10.1016/j.jclinepi.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 7.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catani M, De Milito R, Romagnoli F, Romeo V, Modini C. Laparoscopic colorectal surgery in urgent and emergent settings. Surg Laparosc Endosc Percutan Tech. 2011;21(5):340–343. doi: 10.1097/SLE.0b013e3182318b5c. [DOI] [PubMed] [Google Scholar]
- 9.Dunker MS, Bemelman WA, Slors JF, van Hogezand RA, Ringers J, Gouma DJ. Laparoscopic-assisted vs open colectomy for severe acute colitis in patients with inflammatory bowel disease (IBD): a retrospective study in 42 patients. Surg Endosc. 2000;14(10):911–914. doi: 10.1007/s004640000262. [DOI] [PubMed] [Google Scholar]
- 10.Koh FH, Tan KK, Tsang CB, Koh DC. Laparoscopic versus an open colectomy in an emergency setting: a case-controlled study. Ann Coloproctol. 2013;29(1):12–16. doi: 10.3393/ac.2013.29.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letarte F, Hallet J, Drolet S, Boulanger-Gobeil C, Bouchard A, Gregoire RC, Gagne JP, Thibault C, Bouchard P. Laparoscopic versus open colonic resection for complicated diverticular disease in the emergency setting: a safe choice? A retrospective comparative cohort study. Am J Surg. 2014 doi: 10.1016/j.amjsurg.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Li JC, Ng SS, Lee JF, Yiu RY, Hon SS, Leung WW, Leung KL. Emergency laparoscopic-assisted versus open right hemicolectomy for complicated cecal diverticulitis: a comparative study. J Laparoendosc Adv Surg Tech A. 2009;19(4):479–483. doi: 10.1089/lap.2008.0220. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Li D, Jie Z, Zhang G, Liu Y. Comparative study on therapeutic efficacy between hand-assisted laparoscopic surgery and conventional laparotomy for acute obstructive right-sided colon cancer. J Laparoendosc Adv Surg Tech A. 2015;25(7):548–554. doi: 10.1089/lap.2014.0645. [DOI] [PubMed] [Google Scholar]
- 14.Marcello PW, Milsom JW, Wong SK, Brady K, Goormastic M, Fazio VW. Laparoscopic total colectomy for acute colitis: a case–control study. Dis Colon Rectum. 2001;44(10):1441–1445. doi: 10.1007/BF02234595. [DOI] [PubMed] [Google Scholar]
- 15.Ng SS, Lee JF, Yiu RY, Li JC, Leung WW, Leung KL. Emergency laparoscopic-assisted versus open right hemicolectomy for obstructing right-sided colonic carcinoma: a comparative study of short-term clinical outcomes. World J Surg. 2008;32(3):454–458. doi: 10.1007/s00268-007-9400-0. [DOI] [PubMed] [Google Scholar]
- 16.Odermatt M, Miskovic D, Siddiqi N, Khan J, Parvaiz A. Short- and long-term outcomes after laparoscopic versus open emergency resection for colon cancer: an observational propensity score-matched study. World J Surg. 2013;37(10):2458–2467. doi: 10.1007/s00268-013-2146-y. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe K, Funayama Y, Fukushima K, Shibata C, Takahashi K, Sasaki I. Hand-assisted laparoscopic vs. open subtotal colectomy for severe ulcerative colitis. Dis Colon Rectum. 2009;52(4):640–645. doi: 10.1007/DCR.0b013e31819d47b5. [DOI] [PubMed] [Google Scholar]
- 18.Regenbogen SE, Hardiman KM, Hendren S, Morris AM. Surgery for diverticulitis in the 21st century: a systematic review. JAMA Surg. 2014;149(3):292–303. doi: 10.1001/jamasurg.2013.5477. [DOI] [PubMed] [Google Scholar]
- 19.Schwenter F, Morel P, Gervaz P. Management of obstructive and perforated colorectal cancer. Expert Rev Anticancer Ther. 2010;10(10):1613–1619. doi: 10.1586/era.10.147. [DOI] [PubMed] [Google Scholar]
- 20.Gunnarsson H, Jennische K, Forssell S, Granstrom J, Jestin P, Ekholm A, Olsson LI. Heterogeneity of colon cancer patients reported as emergencies. World J Surg. 2014;38(7):1819–1826. doi: 10.1007/s00268-014-2449-7. [DOI] [PubMed] [Google Scholar]
- 21.Yeo H, Niland J, Milne D, ter Veer A, Bekaii-Saab T, Farma JM, Lai L, Skibber JM, Small W, Jr, Wilkinson N, Schrag D, Weiser MR. Incidence of minimally invasive colorectal cancer surgery at National Comprehensive Cancer Network centers. J Natl Cancer Inst. 2015;107(1):362. doi: 10.1093/jnci/dju362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Z, Jemal A, Lin CC, Hu CY, Chang GJ (2015) Comparative effectiveness of laparoscopy vs open colectomy among nonmetastatic colon cancer patients: an analysis using the national cancer data base. J Natl Cancer Inst 107 (3). doi:10.1093/jnci/dju491 [DOI] [PMC free article] [PubMed]
- 23.Theophilus M, Platell C, Spilsbury K. Long-term survival following laparoscopic and open colectomy for colon cancer: a meta-analysis of randomized controlled trials. Color Dis. 2014;16(3):O75–O81. doi: 10.1111/codi.12483. [DOI] [PubMed] [Google Scholar]
- 24.Vaccaro CA, Rossi GL, Quintana GO, Soriano ER, Vaccarezza H, Rubinstein F. Laparoscopic colorectal resections: a simple predictor model and a stratification risk for conversion to open surgery. Dis Colon Rectum. 2014;57(7):869–874. doi: 10.1097/DCR.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 25.Miskovic D, Ni M, Wyles SM, Tekkis P, Hanna GB. Learning curve and case selection in laparoscopic colorectal surgery: systematic review and international multicenter analysis of 4852 cases. Dis Colon Rectum. 2012;55(12):1300–1310. doi: 10.1097/DCR.0b013e31826ab4dd. [DOI] [PubMed] [Google Scholar]
- 26.Morner ME, Gunnarsson U, Jestin P, Svanfeldt M. The importance of blood loss during colon cancer surgery for long-term survival: an epidemiological study based on a population based register. Ann Surg. 2012;255(6):1126–1128. doi: 10.1097/SLA.0b013e3182512df0. [DOI] [PubMed] [Google Scholar]
- 27.Bailey MB, Davenport DL, Vargas HD, Evers BM, McKenzie SP. Longer operative time: deterioration of clinical outcomes of laparoscopic colectomy versus open colectomy. Dis Colon Rectum. 2014;57(5):616–622. doi: 10.1097/DCR.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 28.Abarca F, Saclarides TJ, Brand MI. Laparoscopic colectomy: complications causing reoperation or emergency room/hospital readmissions. Am Surg. 2011;77(1):65–69. [PubMed] [Google Scholar]
- 29.Rossi G, Vaccarezza H, Vaccaro CA, Mentz RE, Im V, Alvarez A, Quintana GO. Two-day hospital stay after laparoscopic colorectal surgery under an enhanced recovery after surgery (ERAS) pathway. World J Surg. 2013;37(10):2483–2489. doi: 10.1007/s00268-013-2155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy KM, Kwong J, Pitzul KB, Vergis AS, Jackson TD, Urbach DR, Okrainec A. A cost comparison of laparoscopic and open colon surgery in a publicly funded academic institution. Surg Endosc. 2014;28(4):1213–1222. doi: 10.1007/s00464-013-3311-y. [DOI] [PubMed] [Google Scholar]